Magnetic Resonance-Guided High-Intensity Focused Ultrasound Ablation of Uterine Fibroids—Efficiency Assessment with the Use of Dynamic Contrast-Enhanced Magnetic Resonance Imaging and the Potential Role of the Administration of Uterotonic Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Interventions
2.3. Treatment Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Clinical practice. Uterine fibroids. N. Engl. J. Med. 2015, 372, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Ulin, M.; Ali, M.; Chaudhry, Z.T.; Al-Hendy, A.; Yang, Q. Uterine fibroids in menopause and perimenopause. Menopause 2020, 27, 238–242. [Google Scholar] [CrossRef]
- Vilos, G.A.; Allaire, C.; Laberge, P.Y.; Leyland, N.; Special, C. The management of uterine leiomyomas. J. Obstet. Gynaecol. Can. 2015, 37, 157–178. [Google Scholar] [CrossRef] [Green Version]
- Donnez, J.; Dolmans, M.M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef]
- Ciebiera, M.; Lozinski, T. The role of magnetic resonance-guided focused ultrasound in fertility-sparing treatment of uterine fibroids-current perspectives. Ecancermedicalscience 2020, 14, 1034. [Google Scholar] [CrossRef]
- Grube, M.; Neis, F.; Brucker, S.Y.; Kommoss, S.; Andress, J.; Weiss, M.; Hoffmann, S.; Taran, F.A.; Kramer, B. Uterine Fibroids—Current Trends and Strategies. Surg. Technol. Int. 2019, 34, 257–263. [Google Scholar]
- Marsh, E.E.; Al-Hendy, A.; Kappus, D.; Galitsky, A.; Stewart, E.A.; Kerolous, M. Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. J. Womens Health (Larchmt) 2018, 27, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Szkodziak, P.; Pyra, K.; Szkodziak, F.; Krzyzanowski, J.; Czuczwar, P.; Wozniak, S.; Jargiello, T.; Paszkowski, T. The Lublin Protocol of the Uterine Arteries Embolization in the Treatment of Symptomatic Uterine Fibroids. J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Stewart, E.A.; Rabinovici, J.; Tempany, C.M.; Inbar, Y.; Regan, L.; Gostout, B.; Hesley, G.; Kim, H.S.; Hengst, S.; Gedroyc, W.M. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil. Steril. 2006, 85, 22–29. [Google Scholar] [CrossRef]
- Schlesinger, D.; Benedict, S.; Diederich, C.; Gedroyc, W.; Klibanov, A.; Larner, J. MR-guided focused ultrasound surgery, present and future. Med. Phys. 2013, 40, 080901. [Google Scholar] [CrossRef]
- Verpalen, I.M.; Anneveldt, K.J.; Nijholt, I.M.; Schutte, J.M.; Dijkstra, J.R.; Franx, A.; Bartels, L.W.; Moonen, C.T.W.; Edens, M.A.; Boomsma, M.F. Magnetic resonance-high intensity focused ultrasound (MR-HIFU) therapy of symptomatic uterine fibroids with unrestrictive treatment protocols: A systematic review and meta-analysis. Eur. J. Radiol. 2019, 120, 108700. [Google Scholar] [CrossRef]
- Copelan, A.; Hartman, J.; Chehab, M.; Venkatesan, A.M. High-Intensity Focused Ultrasound: Current Status for Image-Guided Therapy. Semin. Intervent. Radiol. 2015, 32, 398–415. [Google Scholar] [CrossRef] [Green Version]
- Kroncke, T.; David, M.; participants of the Consensus, M. Magnetic Resonance Guided Focused Ultrasound for Fibroid Treatment—Results of the Second Radiological Gynecological Expert Meeting. Geburtshilfe Frauenheilkd 2015, 75, 436–438. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- Clasen, S.; Pereira, P.L. Magnetic resonance guidance for radiofrequency ablation of liver tumors. J. Magn. Reson. Imaging 2008, 27, 421–433. [Google Scholar] [CrossRef]
- Campbell-Washburn, A.E.; Tavallaei, M.A.; Pop, M.; Grant, E.K.; Chubb, H.; Rhode, K.; Wright, G.A. Real-time MRI guidance of cardiac interventions. J. Magn. Reson. Imaging 2017, 46, 935–950. [Google Scholar] [CrossRef]
- Lozinski, T.; Filipowska, J.; Ludwin, A.; Ciebiera, M. The outcome of magnetic resonance-guided high-intensity ultrasound for clinically symptomatic submucosal uterine fibroid performed accidentally in very early pregnancy: A case report. Int. J. Hyperth. 2019, 36, 975–979. [Google Scholar] [CrossRef]
- Duc, N.M.; Keserci, B. Review of influential clinical factors in reducing the risk of unsuccessful MRI-guided HIFU treatment outcome of uterine fibroids. Diagn. Interv. Radiol. 2018, 24, 283–291. [Google Scholar] [CrossRef]
- Funaki, K.; Fukunishi, H.; Funaki, T.; Sawada, K.; Kaji, Y.; Maruo, T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: Relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am. J. Obstet. Gynecol. 2007, 196, 184.e1. [Google Scholar] [CrossRef]
- Fan, H.J.; Zhang, C.; Lei, H.T.; Cun, J.P.; Zhao, W.; Huang, J.Q.; Zhai, Y. Ultrasound-guided high-intensity focused ultrasound in the treatment of uterine fibroids. Medicine (Baltimore) 2019, 98, e14566. [Google Scholar] [CrossRef]
- Sainio, T.; Saunavaara, J.; Komar, G.; Mattila, S.; Otonkoski, S.; Joronen, K.; Perheentupa, A.; Blanco Sequeiros, R. Feasibility of apparent diffusion coefficient in predicting the technical outcome of MR-guided high-intensity focused ultrasound treatment of uterine fibroids—A comparison with the Funaki classification. Int. J. Hyperth. 2021, 38, 85–94. [Google Scholar] [CrossRef]
- Lozinski, T.; Filipowska, J.; Krol, P.; Kubaty, A.; Wegrzyn, P. Oxytocin Administration in High-Intensity Focused Ultrasound Treatment of Myomata. Biomed. Res. Int. 2018, 2018, 7518026. [Google Scholar] [CrossRef] [Green Version]
- Otonkoski, S.; Sainio, T.; Komar, G.; Suomi, V.; Saunavaara, J.; Blanco Sequeiros, R.; Perheentupa, A.; Joronen, K. Oxytocin selectively reduces blood flow in uterine fibroids without an effect on myometrial blood flow: A dynamic contrast enhanced MRI evaluation. Int. J. Hyperth. 2020, 37, 1293–1300. [Google Scholar] [CrossRef]
- Jeong, J.H.; Hong, G.P.; Kim, Y.R.; Ha, J.E.; Lee, K.S. Clinical Consideration of Treatment to Ablate Uterine Fibroids with Magnetic Resonance Imaging-guided High Intensity Focused Ultrasound (MRgFUS): Sonalleve. J. Menopausal. Med. 2016, 22, 94–107. [Google Scholar] [CrossRef] [Green Version]
- Lozinski, T.; Ludwin, A.; Filipowska, J.; Zgliczynska, M.; Wegrzyn, P.; Kluz, T.; Ciebiera, M. Oxytocin and misoprostol with diclofenac in the preparation for MR-HIFU treatment of symptomatic uterine fibroids: A prospective cohort study. Ultrasound Med. Biol. 2021, 47, 1573–1585. [Google Scholar] [CrossRef]
- Yu, S.C.; Cheung, E.C.; Leung, V.Y.; Fung, L.W. Oxytocin-Augmented and Non-Sedating High-Intensity-Focused Ultrasound (HIFU) for Uterine Fibroids Showed Promising Outcome As Compared To HIFU Alone or Uterine Artery Embolization. Ultrasound Med. Biol. 2019, 45, 3207–3213. [Google Scholar] [CrossRef]
- Samy, A.; Raslan, A.N.; Talaat, B.; El Lithy, A.; El Sharkawy, M.; Sharaf, M.F.; Hussein, A.H.; Amin, A.H.; Ibrahim, A.M.; Elsherbiny, W.S.; et al. Perioperative nonhormonal pharmacological interventions for bleeding reduction during open and minimally invasive myomectomy: A systematic review and network meta-analysis. Fertil. Steril. 2020, 113, 224–233.e6. [Google Scholar] [CrossRef]
- Allen, R.; O’Brien, B.M. Uses of misoprostol in obstetrics and gynecology. Rev. Obstet. Gynecol. 2009, 2, 159–168. [Google Scholar]
- Gordon, Y.; Partovi, S.; Muller-Eschner, M.; Amarteifio, E.; Bauerle, T.; Weber, M.A.; Kauczor, H.U.; Rengier, F. Dynamic contrast-enhanced magnetic resonance imaging: Fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc. Diagn. Ther. 2014, 4, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Macvicar, D.A.; Ayton, V.; Husband, J.E. The role of intravenous contrast enhancement in magnetic resonance imaging of prostatic carcinoma. Clin. Radiol. 1995, 50, 601–606. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Akin, O.; Hricak, H. Dynamic contrast-enhanced magnetic resonance imaging of prostate cancer: A review of current methods and applications. World J. Radiol. 2017, 9, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, H.; DeMartini, W.B.; Lee, A.Y.; Partridge, S.C.; Peacock, S.; Lehman, C.D. Accuracy of 3 T versus 1.5 T breast MRI for pre-operative assessment of extent of disease in newly diagnosed DCIS. Eur. J. Radiol. 2015, 84, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.; Bassett, K.; Foerster, t.; Spry, C.; Tong, L. 1.5 tesla magnetic resonance imaging scanners compared with 3.0 tesla magnetic resonance imaging scanners: Systematic review of clinical effectiveness. CADTH Technol. Overv. 2012, 2, e2201. [Google Scholar]
- Li, S.; Wu, P.H. Magnetic resonance image-guided versus ultrasound-guided high-intensity focused ultrasound in the treatment of breast cancer. Chin. J. Cancer 2013, 32, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Larsen, V.A.; Simonsen, H.J.; Law, I.; Larsson, H.B.; Hansen, A.E. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 2013, 55, 361–369. [Google Scholar] [CrossRef]
- Geraci, L.; Napoli, A.; Catalano, C.; Midiri, M.; Gagliardo, C. Magnetic Resonance Imaging-Guided Focused Ultrasound Surgery for the Treatment of Symptomatic Uterine Fibroids. Case Rep. Radiol. 2017, 2017, 2520989. [Google Scholar] [CrossRef]
- Rueff, L.E.; Raman, S.S. Clinical and Technical Aspects of MR-Guided High Intensity Focused Ultrasound for Treatment of Symptomatic Uterine Fibroids. Semin. Interv. Radiol. 2013, 30, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Ueda, H.; Togashi, K.; Konishi, I.; Kataoka, M.L.; Koyama, T.; Fujiwara, T.; Kobayashi, H.; Fujii, S.; Konishi, J. Unusual appearances of uterine leiomyomas: MR imaging findings and their histopathologic backgrounds. Radiographics 1999, 19, S131–S145. [Google Scholar] [CrossRef]
- Suomi, V.; Komar, G.; Sainio, T.; Joronen, K.; Perheentupa, A.; Blanco Sequeiros, R. Comprehensive feature selection for classifying the treatment outcome of high-intensity ultrasound therapy in uterine fibroids. Sci. Rep. 2019, 9, 10907. [Google Scholar] [CrossRef] [Green Version]
- Jondal, D.E.; Wang, J.; Chen, J.; Gorny, K.R.; Felmlee, J.; Hesly, G.; Laughlin-Tommaso, S.; Stewart, E.A.; Ehman, R.; Woodrum, D.A. Uterine fibroids: Correlations between MRI appearance and stiffness via magnetic resonance elastography. Abdom. Radiol. 2018, 43, 1456–1463. [Google Scholar] [CrossRef]
- Demirel, H.C.; Davis, J.W. Multiparametric magnetic resonance imaging: Overview of the technique, clinical applications in prostate biopsy and future directions. Turk. J. Urol. 2018, 44, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Setzen, R.; Liu, Z.; Liu, Y.; Xie, B.; Aili, A.; Zhang, L. High intensity focused ultrasound treatment of adenomyosis: The relationship between the features of magnetic resonance imaging on T2 weighted images and the therapeutic efficacy. Eur. J. Radiol. 2017, 89, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jin, C.; Liang, T.; Li, X.; Wang, R.; Zhang, Y.; Yang, J. Magnetic resonance-guided high-intensity focused ultrasound of uterine fibroids: Whole-tumor quantitative perfusion for prediction of immediate ablation response. Acta Radiol. 2020, 61, 1125–1133. [Google Scholar] [CrossRef]
- Malone, C.D.; Banerjee, A.; Alley, M.T.; Vasanawala, S.S.; Roberts, A.C.; Hsiao, A. Pelvic Blood Flow Predicts Fibroid Volume and Embolic Required for Uterine Fibroid Embolization: A Pilot Study With 4D Flow MR Angiography. AJR Am. J. Roentgenol. 2018, 210, 189–200. [Google Scholar] [CrossRef]
- Zlotnik, E.; Lorenzo Messina, M.; Nasser, F.; Affonso, B.B.; Baroni, R.H.; Wolosker, N.; Baracat, E.C. Predictive factors for pelvic magnetic resonance in response to arterial embolization of a uterine leiomyoma. Clinics (Sao Paulo) 2014, 69, 185–189. [Google Scholar] [CrossRef]
- Holdsworth-Carson, S.J.; Zhao, D.; Cann, L.; Bittinger, S.; Nowell, C.J.; Rogers, P.A. Differences in the cellular composition of small versus large uterine fibroids. Reproduction 2016, 152, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Swe, T.T.; Onitsuka, H.; Kawamoto, K.; Ueyama, T.; Tsuruchi, N.; Masuda, K. Uterine leiomyoma: Correlation between signal intensity on magnetic resonance imaging and pathologic characteristics. Radiat. Med. 1992, 10, 235–242. [Google Scholar]
- Giordano, M.A.; Gutierrez, G.; Rinaldi, C. Fundamental solutions to the bioheat equation and their application to magnetic fluid hyperthermia. Int. J. Hyperth. 2010, 26, 475–484. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lim, H.K.; Kim, J.H.; Rhim, H.; Park, B.K.; Keserci, B.; Kohler, M.O.; Bae, D.S.; Kim, B.G.; Lee, J.W.; et al. Dynamic contrast-enhanced magnetic resonance imaging predicts immediate therapeutic response of magnetic resonance-guided high-intensity focused ultrasound ablation of symptomatic uterine fibroids. Investig. Radiol. 2011, 46, 639–647. [Google Scholar] [CrossRef]
- Wei, C.; Fang, X.; Wang, C.B.; Chen, Y.; Xu, X.; Dong, J.N. The predictive value of quantitative DCE metrics for immediate therapeutic response of high-intensity focused ultrasound ablation (HIFU) of symptomatic uterine fibroids. Abdom. Radiol. 2018, 43, 2169–2175. [Google Scholar] [CrossRef]
- Madhuranthakam, A.J.; Yuan, Q.; Pedrosa, I. Quantitative Methods in Abdominal MRI: Perfusion Imaging. Top. Magn. Reson. Imaging 2017, 26, 251–258. [Google Scholar] [CrossRef]
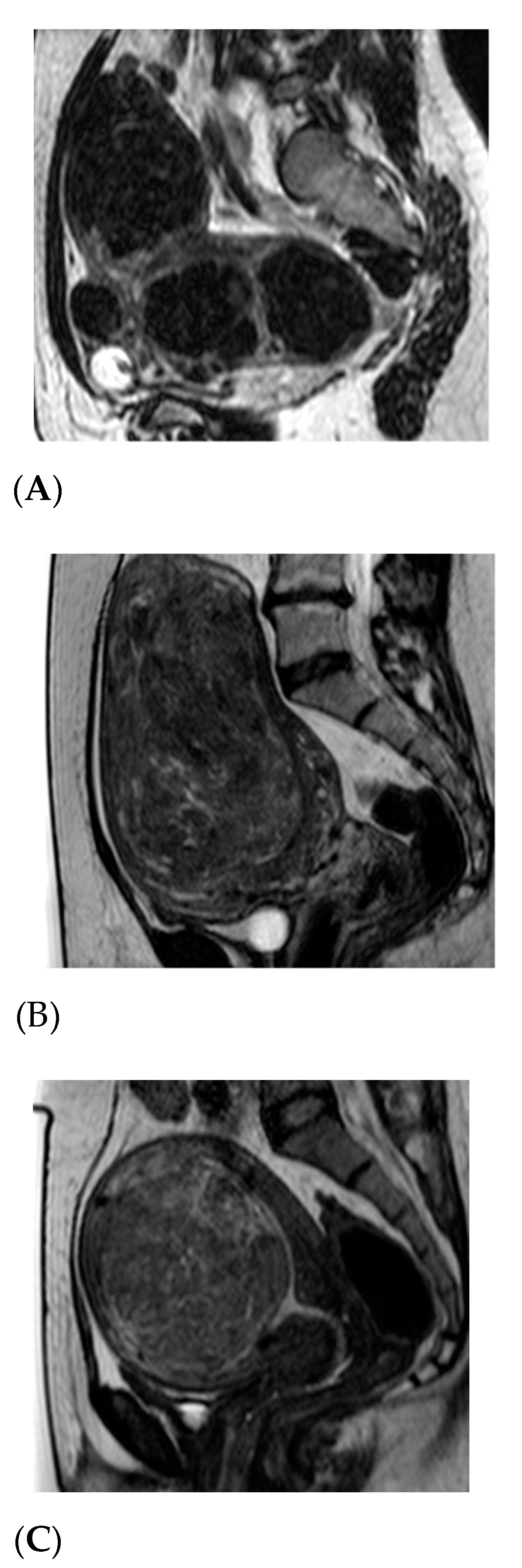



| Inclusion Criteria | Exclusion Criteria |
|---|---|
| age 20–50 years | pregnancy |
| UF measuring 1–13 cm in diameter | calcified or predominantly degenerated UFs |
| blood platelet count ≥ 100,000/mm3 | obese patients with a thick abdominal wall fat layer |
| serum creatinine level ≤ 2.0 mg/dL | the presence of a scar in the sonication path |
| MRI criteria (Funaki type I and II only) optimal acoustic window, i.e., without intestine loops in the sonication path) | contraindications to MRI |
| maximal number of UFs = 2 | pedunculated UFs |
| more than 12 cm from the transducer to the front wall of UF | |
| too short distance from the sacral bone < 20 mm | |
| The location of the UFs on the back wall of the uterus and/or in direct contact with the rectum |
| Parameter | T2 Weighted | Dynamic Contrast Enhanced (DCE) Curves |
|---|---|---|
| Acquisition plane | axial, sagittal | turbo field echo imaging |
| X averages | Non-steroidal anti-inflammatory (NSA)1 | NSA1 |
| Slice thickness (mm) | 4 | 2.4 |
| Slice gap (mm) | 0.5 | −1.2 |
| Acquisition duration | 01 min 00 s | 4 min 31 s |
| Phase-encoding direction | anterior-posterior | right-left |
| Field of view (mm) | 230 × 197 | 320 × 368 |
| Acquisition matrix | 256 × 177 | 320–275 |
| Repetition time/echo time (ms) | 1505/80 | 3.2/1.53 |
| Flip angle (degree) | 120 | 10 |
| Bandwidth (Hz/pixel) | 375 | 723.4 |
| N | Mean | SD | Min | Max | Q25 | Median | Q75 | CV | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age [n] | Control | 155 | 36.19 | 5.07 | 19.00 | 46.00 | 32.00 | 37.00 | 40.00 | 14.0% |
| Misoprostol | 56 | 35.98 | 4.81 | 26.00 | 45.00 | 32.50 | 36.50 | 40.00 | 13.4% | |
| Oxytocin | 71 | 35.49 | 4.63 | 26.00 | 48.00 | 33.00 | 36.00 | 38.00 | 13.0% | |
| BMI [kg/m2] | Control | 154 | 23.26 | 3.47 | 17.93 | 34.72 | 20.58 | 22.90 | 25.51 | 14.9% |
| Misoprostol | 56 | 22.87 | 2.89 | 16.65 | 31.31 | 21.23 | 22.27 | 24.21 | 12.6% | |
| Oxytocin | 71 | 23.48 | 4.12 | 15.76 | 38.30 | 20.28 | 22.65 | 27.06 | 17.5% | |
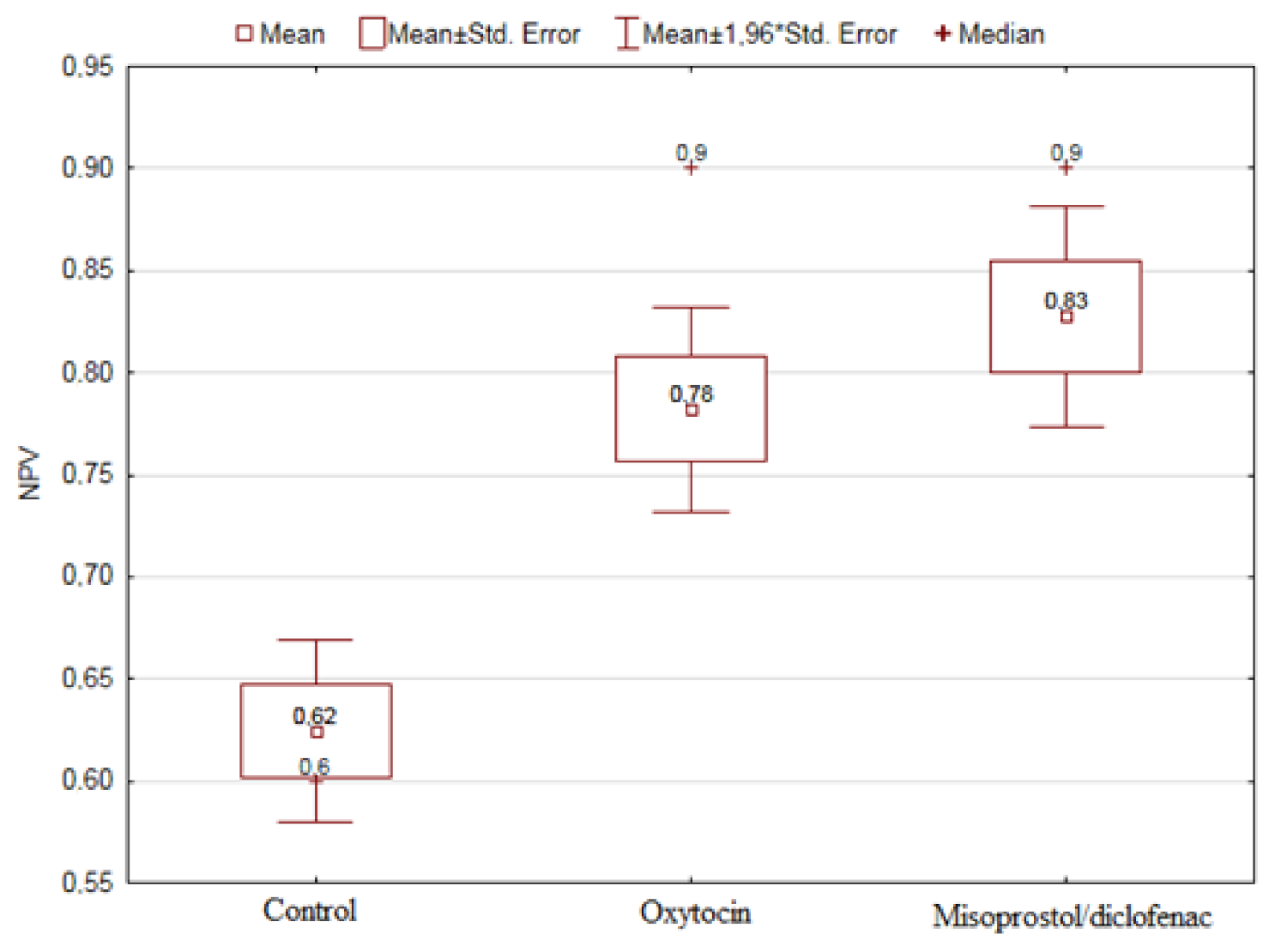
| NPV [%] | Control | 116 | 62.47 | 24.63 | 10.00 | 100.00 | 50.00 | 60.00 | 80.00 | 39.4% |
| Misoprostol | 49 | 82.76 | 19.20 | 25.00 | 100.00 | 70.00 | 90.00 | 100.00 | 23.2% | |
| Oxytocin | 67 | 78.21 | 20.94 | 20.00 | 100.00 | 70.00 | 90.00 | 90.00 | 26.8% | |
| MRI volume [cm3] | Control | 153 | 74.62 | 88.29 | 2.13 | 586.50 | 18.60 | 42.24 | 99.10 | 118.3% |
| Misoprostol | 54 | 96.04 | 113.36 | 4.64 | 625.40 | 20.97 | 56.30 | 129.16 | 118.0% | |
| Oxytocin | 71 | 92.77 | 102.50 | 2.76 | 550.62 | 18.65 | 57.39 | 139.57 | 110.5% |
| N | Mean | Median | Min | Max | Q25 | Q75 | SD | CV | |
|---|---|---|---|---|---|---|---|---|---|
| Volume change (6 months) | 82 | 38 | 36 | −26 | 96 | 20 | 60 | 27 | 72.03 |
| Max. temperature [°C] | 231 | 78.53 | 72.60 | 57.80 | 178.00 | 69.00 | 82.00 | 17.29 | 22.02 |
| Max. power [W] | 231 | 179.45 | 180.00 | 100.00 | 270.00 | 160.00 | 200.00 | 33.89 | 18.89 |
| Min. time to optimal temperature [sec] | 146 | 9.05 | 8.50 | 1.00 | 26.00 | 5.00 | 13.00 | 5.05 | 55.72 |
| Funaki Type I | Funaki Type II | Total | |
|---|---|---|---|
| Washout absent | 147 (61.5%) | 92 (38.5%) | 239 (85%) |
| Washout present | 24 (55%) | 20 (45%) | 44 (15%) |
| NPV | Enhancement Curve | p-Value | |
|---|---|---|---|
| No Washout | Washout | ||
| <50% | 25 (74%) | 9 (26%) | 0.0446 |
| >50% | 172 (87%) | 26 (13%) | |
| Characteristic | Group | Mean | Median | p-Value |
|---|---|---|---|---|
| BMI [kg/m2] | Without washout | 23.22 | 22.65 | 0.7301 |
| Washout | 23.33 | 23.00 | ||
| Age [n] | Without washout | 35.88 | 37.00 | 0.6563 |
| Washout | 36,47 | 36.00 | ||
| Volume change after 6 months [%] | Without washout | 0.35 | 0.31 | 0.0085 |
| Washout | 0.54 | 0.63 | ||
| Max. temp [ºC] | Without washout | 79.16 | 72.20 | 0.9286 |
| Washout | 78.17 | 73.00 | ||
| Max. used power [W] | Without washout | 182.33 | 180.00 | 0.0407 |
| Washout | 170.28 | 170.00 | ||
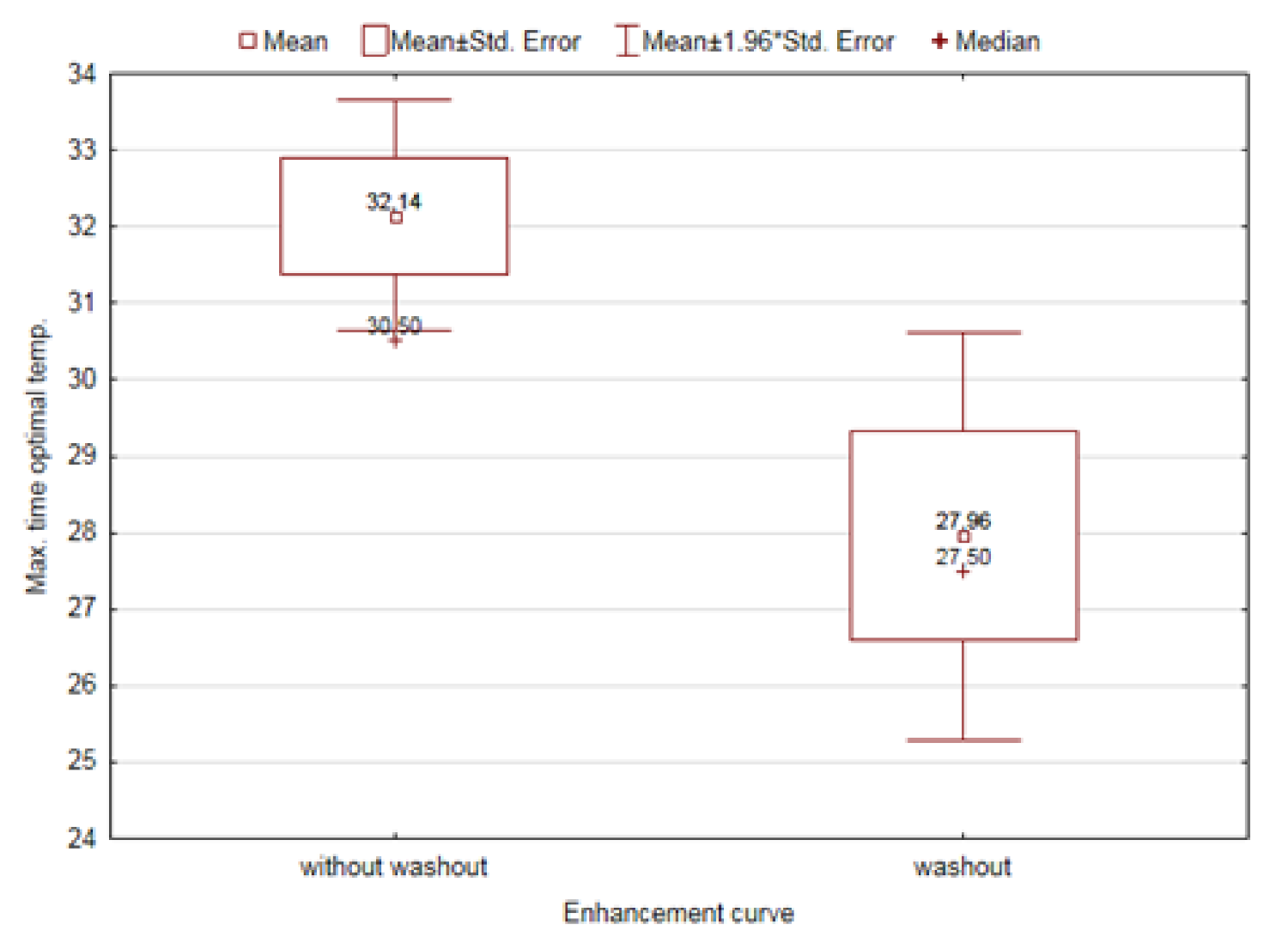
| Max. time to optimal temperature [sec] | Without washout | 32.14 | 30.50 | 0.0186 |
| Washout | 27.96 | 27.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łoziński, T.; Ciebiera, M.; Łuczyńska, E.; Filipowska, J.; Czekierdowski, A. Magnetic Resonance-Guided High-Intensity Focused Ultrasound Ablation of Uterine Fibroids—Efficiency Assessment with the Use of Dynamic Contrast-Enhanced Magnetic Resonance Imaging and the Potential Role of the Administration of Uterotonic Drugs. Diagnostics 2021, 11, 715. https://doi.org/10.3390/diagnostics11040715
Łoziński T, Ciebiera M, Łuczyńska E, Filipowska J, Czekierdowski A. Magnetic Resonance-Guided High-Intensity Focused Ultrasound Ablation of Uterine Fibroids—Efficiency Assessment with the Use of Dynamic Contrast-Enhanced Magnetic Resonance Imaging and the Potential Role of the Administration of Uterotonic Drugs. Diagnostics. 2021; 11(4):715. https://doi.org/10.3390/diagnostics11040715
Chicago/Turabian StyleŁoziński, Tomasz, Michał Ciebiera, Elżbieta Łuczyńska, Justyna Filipowska, and Artur Czekierdowski. 2021. "Magnetic Resonance-Guided High-Intensity Focused Ultrasound Ablation of Uterine Fibroids—Efficiency Assessment with the Use of Dynamic Contrast-Enhanced Magnetic Resonance Imaging and the Potential Role of the Administration of Uterotonic Drugs" Diagnostics 11, no. 4: 715. https://doi.org/10.3390/diagnostics11040715
APA StyleŁoziński, T., Ciebiera, M., Łuczyńska, E., Filipowska, J., & Czekierdowski, A. (2021). Magnetic Resonance-Guided High-Intensity Focused Ultrasound Ablation of Uterine Fibroids—Efficiency Assessment with the Use of Dynamic Contrast-Enhanced Magnetic Resonance Imaging and the Potential Role of the Administration of Uterotonic Drugs. Diagnostics, 11(4), 715. https://doi.org/10.3390/diagnostics11040715






