Deep Active Learning for Automatic Segmentation of Maxillary Sinus Lesions Using a Convolutional Neural Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets and Pre-Processing
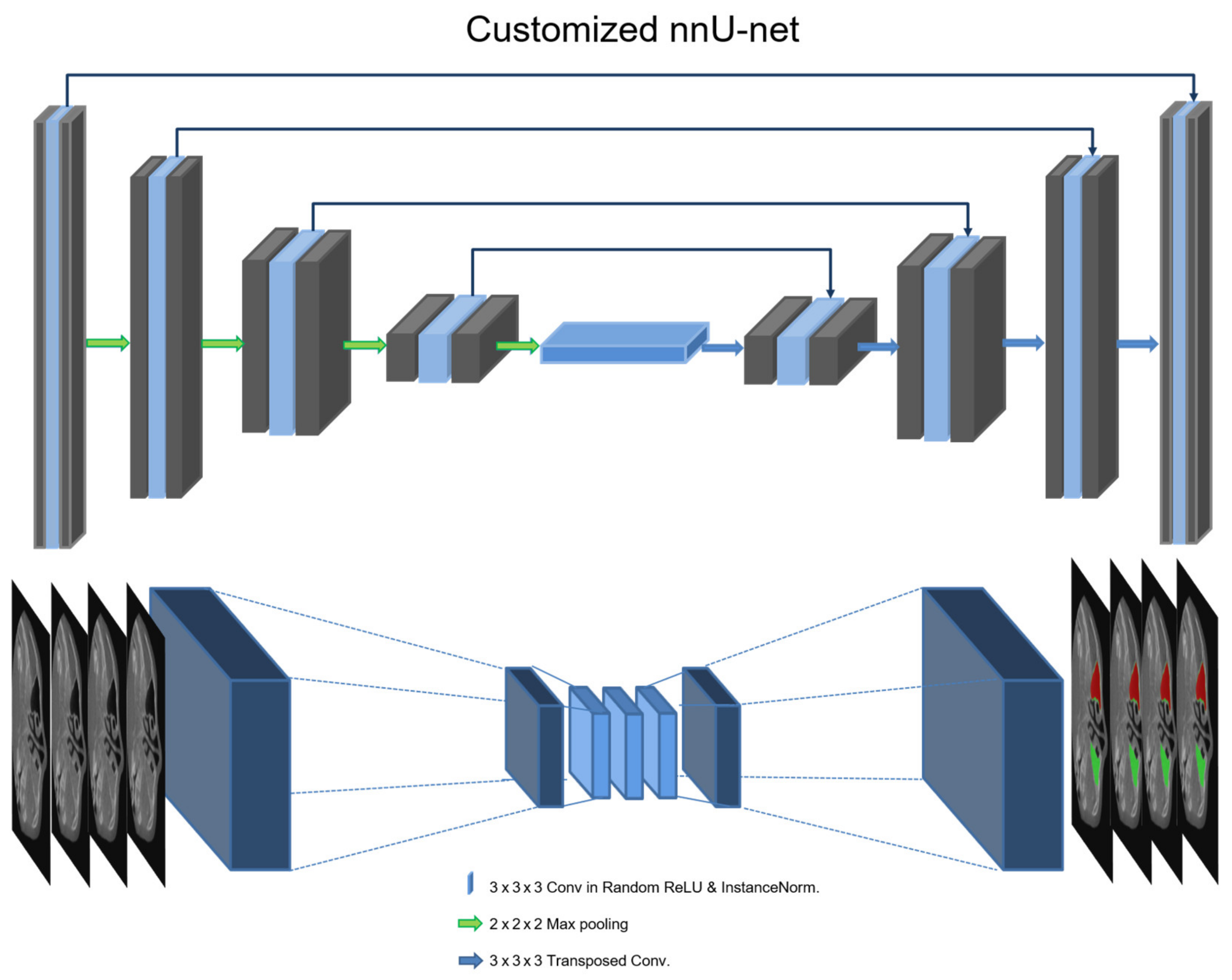
2.2. Training Architecture
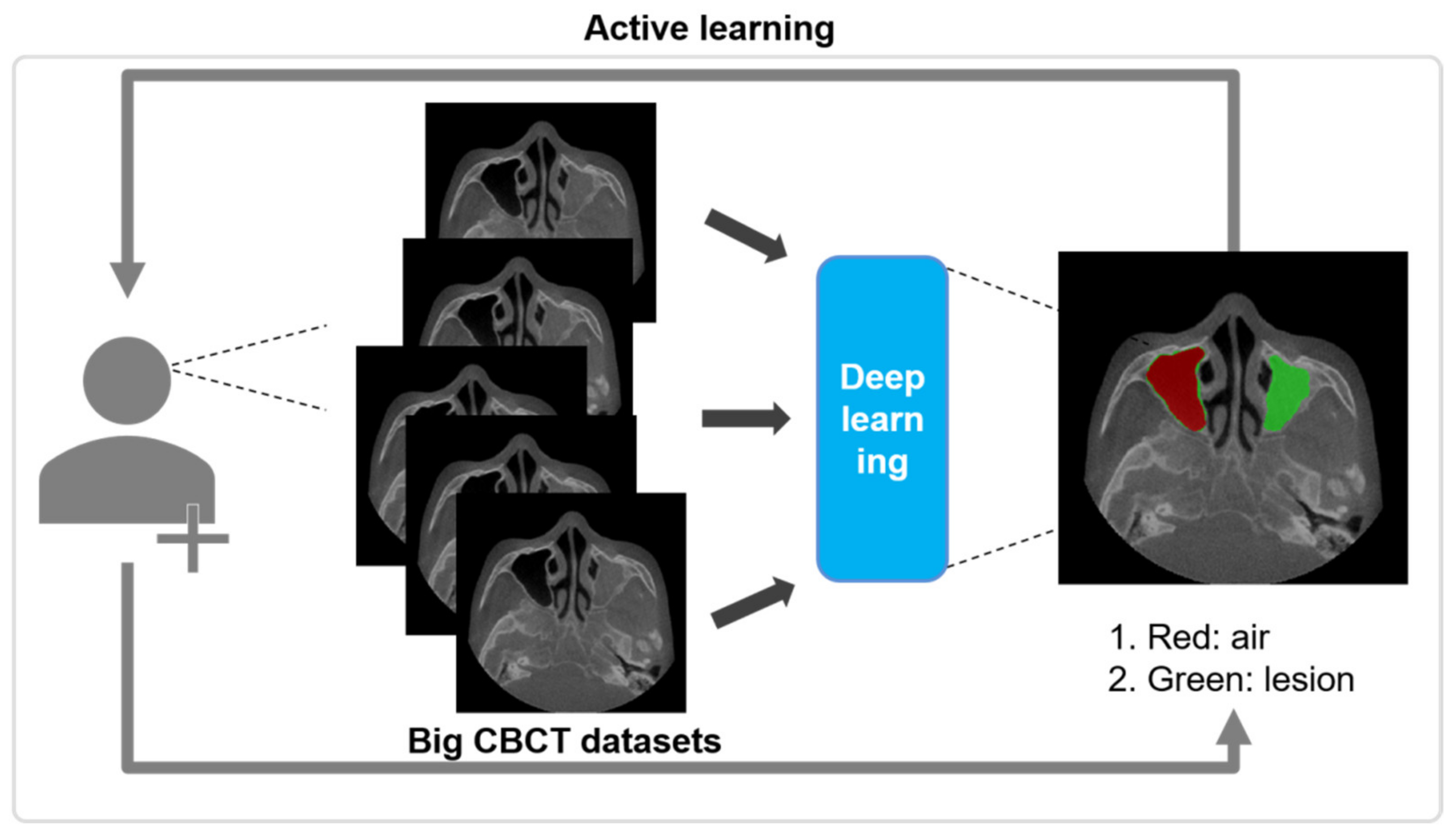
2.3. Active Learning
2.4. Experimental Setup
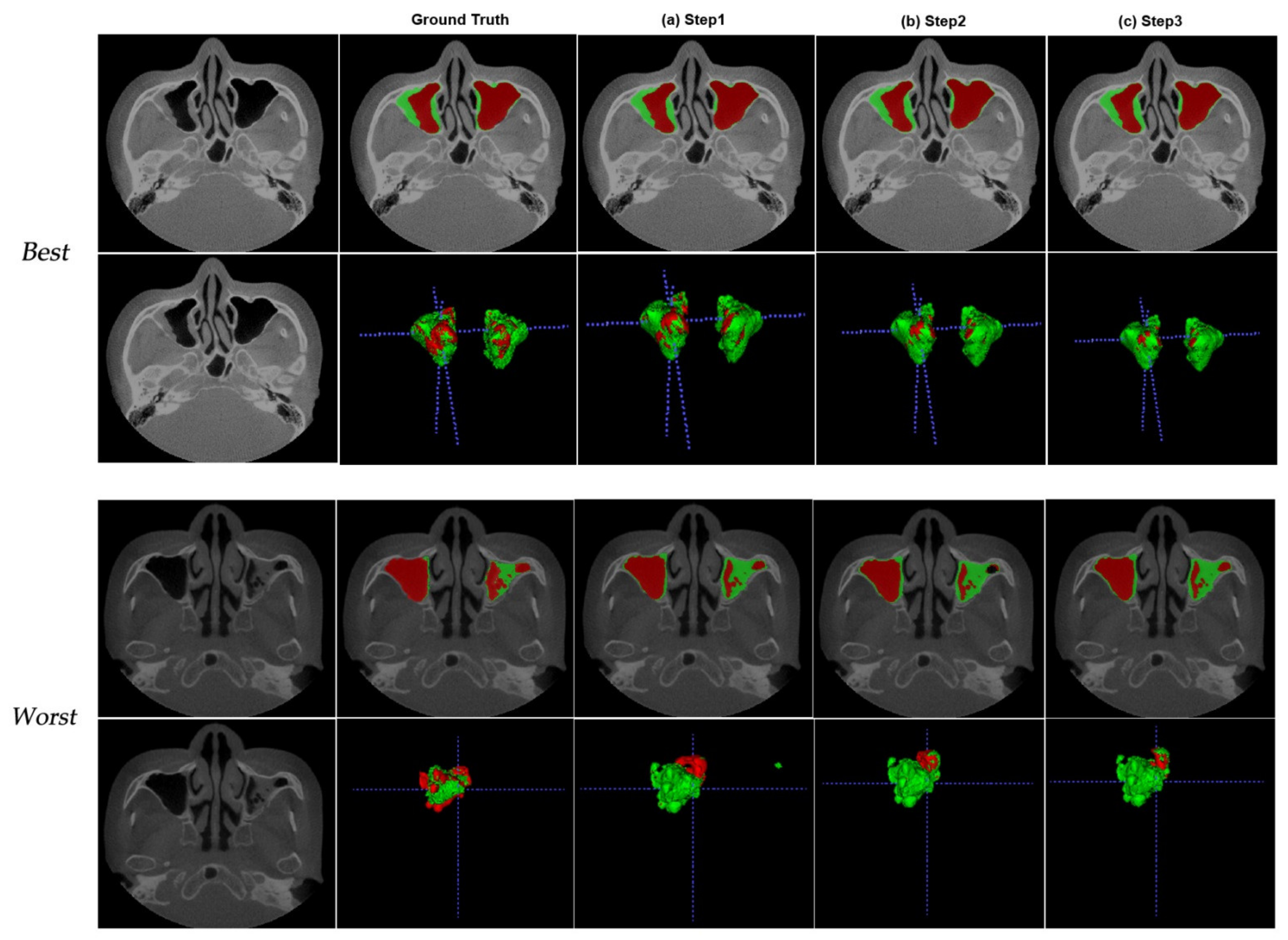
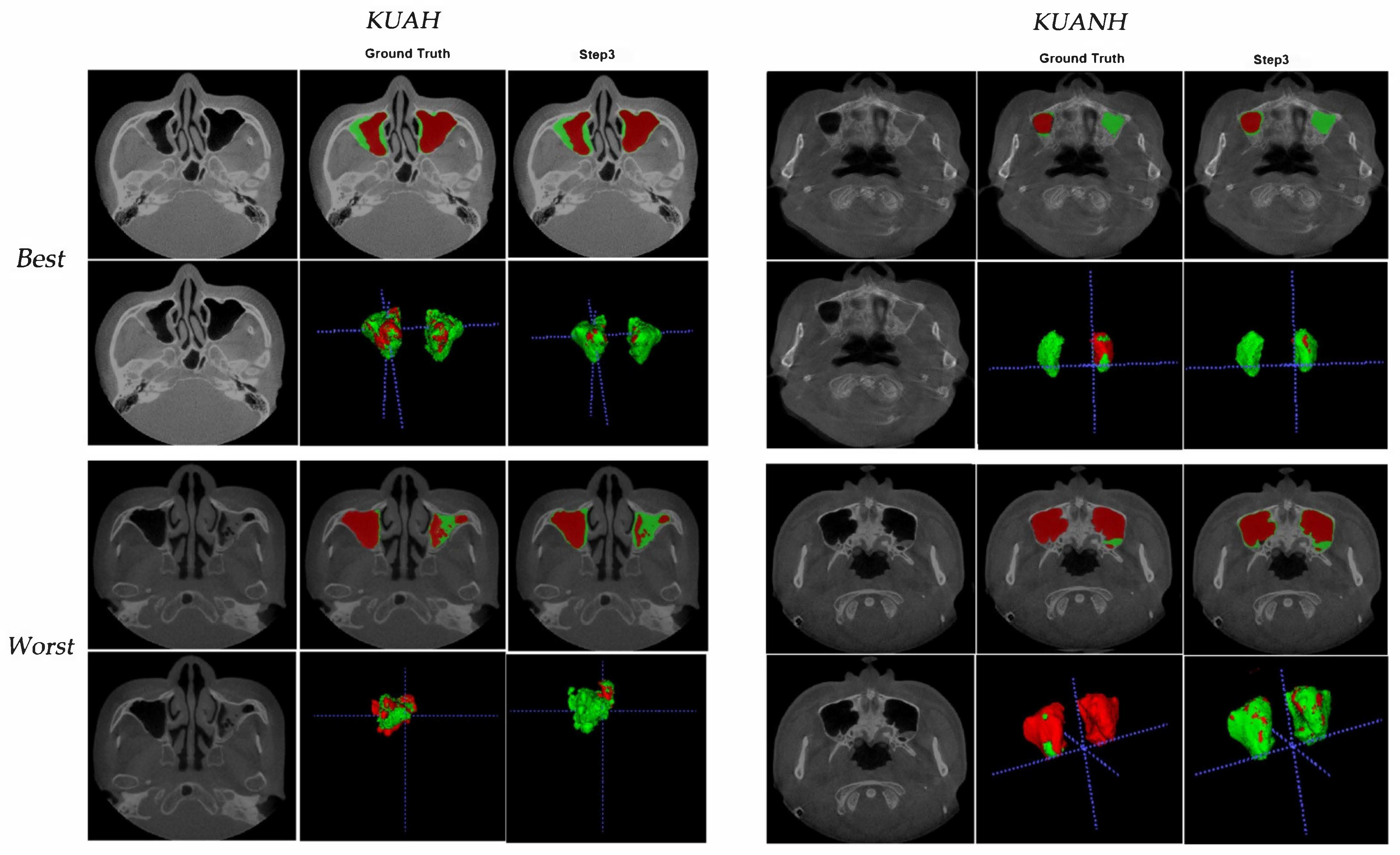
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shalbaf, A.; Bagherzadeh, S.; Maghsoudi, A. Transfer learning with deep convolutional neural network for automated detection of schizophrenia from EEG signals. Phys. Eng. Sci. Med. 2020, 43, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Nogay, H.S.; Adeli, H. Detection of Epileptic Seizure Using Pretrained Deep Convolutional Neural Network and Transfer Learning. Eur. Neurol. 2020, 83, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, K.; Ham, S.; Park, B.; Lee, S.; Hong, D.; Kim, G.B.; Kyung, Y.S.; Kim, C.-S.; Kim, N. Active learning for accuracy enhancement of semantic segmentation with CNN-corrected label curations: Evaluation on kidney segmentation in abdominal CT. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Jung, S.-K.; Ryu, J.-J.; Shin, S.-W.; Choi, J. Evaluation of Transfer Learning with Deep Convolutional Neural Networks for Screening Osteoporosis in Dental Panoramic Radiographs. J. Clin. Med. 2020, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Men, K.; Chen, X.; Zhang, Y.; Zhang, T.; Dai, J.; Yi, J.; Li, Y. Deep Deconvolutional Neural Network for Target Segmentation of Nasopharyngeal Cancer in Planning Computed Tomography Images. Front. Oncol. 2017, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Samala, R.K.; Chan, H.-P.; Hadjiiski, L.; Helvie, M.A.; Wei, J.; Cha, K. Mass detection in digital breast tomosynthesis: Deep convolutional neural network with transfer learning from mammography. Med. Phys. 2016, 43, 6654–6666. [Google Scholar] [CrossRef]
- Kwak, G.H.; Kwak, E.-J.; Song, J.M.; Park, H.R.; Jung, Y.-H.; Cho, B.-H.; Hui, P.; Hwang, J.J. Automatic mandibular canal detection using a deep convolutional neural network. Sci. Rep. 2020, 10, 5711. [Google Scholar] [CrossRef] [PubMed]
- Jaskari, J.; Sahlsten, J.; Järnstedt, J.; Mehtonen, H.; Karhu, K.; Sundqvist, O.; Hietanen, A.; Varjonen, V.; Mattila, V.; Kaski, K. Deep Learning Method for Mandibular Canal Segmentation in Dental Cone Beam Computed Tomography Volumes. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Lee, K.-S.; Ryu, J.-J.; Jang, H.S.; Lee, D.-Y.; Jung, S.-K. Deep Convolutional Neural Networks Based Analysis of Cephalometric Radiographs for Differential Diagnosis of Orthognathic Surgery Indications. Appl. Sci. 2020, 10, 2124. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lee, S.-J.; Yong, T.-H.; Shin, N.-Y.; Jang, B.-G.; Kim, J.-E.; Huh, K.-H.; Lee, S.-S.; Heo, M.-S.; Choi, S.-C.; et al. Deep Learning Hybrid Method to Automatically Diagnose Periodontal Bone Loss and Stage Periodontitis. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.-S.; Song, I.-S.; Jung, K.-H. DeNTNet: Deep Neural Transfer Network for the detection of periodontal bone loss using panoramic dental radiographs. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Kwon, O.; Yong, T.-H.; Kang, S.-R.; Kim, J.-E.; Huh, K.-H.; Heo, M.-S.; Lee, S.-S.; Choi, S.-C.; Yi, W.-J. Automatic diagnosis for cysts and tumors of both jaws on panoramic radiographs using a deep convolution neural network. Dentomaxillofacial Radiol. 2020, 49, 20200185. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Lee, K.M.; Kim, E.J.; Lee, J.S. Improvement diagnostic accuracy of sinusitis recognition in paranasal sinus X-ray using multiple deep learning models. Quant. Imaging Med. Surg. 2019, 9, 942–951. [Google Scholar] [CrossRef]
- Murata, M.; Ariji, Y.; Ohashi, Y.; Kawai, T.; Fukuda, M.; Funakoshi, T.; Kise, Y.; Nozawa, M.; Katsumata, A.; Fujita, H.; et al. Deep-learning classification using convolutional neural network for evaluation of maxillary sinusitis on panoramic radiography. Oral Radiol. 2019, 35, 301–307. [Google Scholar] [CrossRef]
- Qin, Y.; Kamnitsas, K.; Ancha, S.; Nanavati, J.; Cottrell, G.; Criminisi, A.; Nori, A. Autofocus Layer for Semantic Segmentation. Lect. Notes Comput. Sci. 2018, 603–611. [Google Scholar]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef]
- Baid, U.; Talbar, S.; Rane, S.; Gupta, S.; Thakur, M.H.; Moiyadi, A.; Sable, N.; Akolkar, M.; Mahajan, A. A Novel Approach for Fully Automatic Intra-Tumor Segmentation With 3D U-Net Architecture for Gliomas. Front. Comput. Neurosci. 2020, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- U-GAT-IT: Unsupervised Generative Attentional Networks with Adaptive Layer-Instance Normalization for Image-to-Image Translation; arXiv:1907.10830 [cs.CV]; Computer Vision and Pattern Recognition (cs.CV); Image and Video Processing (eess.IV); Cornell University: Ithaca, NY, USA, 2019.
- Kervadec, H.; Bouchtiba, J.; Desrosiers, C.; Granger, E.; Dolz, J.; Ben Ayed, I. Boundary loss for highly unbalanced segmentation. Med. Image Anal. 2021, 67, 101851. [Google Scholar] [CrossRef]
- Sourati, J.; Gholipour, A.; Dy, J.G.; Kurugol, S.; Warfield, S.K. Active Deep learning with fisher information for patch-wise semantic segmentation. Deep. Learn. Med. Image Anal. Multimodal Learn. Clin. Decis. Support 2018, 11045, 83–91. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, Z.; Cobzas, D.; Jagersand, M.; Jaremko, J.L.; Zhang, Z. Segmentation-by-detection: A cascade network for volumetric medical image segmentation. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; Volume 2, pp. 1356–1359. [Google Scholar] [CrossRef]
- Roth, H.R.; Oda, H.; Zhou, X.; Shimizu, N.; Yang, Y.; Hayashi, Y.; Oda, M.; Fujiwara, M.; Misawa, K.; Mori, K. An application of cascaded 3D fully convolutional networks for medical image segmentation. Comput. Med. Imaging Graph. 2018, 66, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Chen, J.; Zhang, S.; Chen, D.Z. Suggestive Annotation: A Deep Active Learning Framework for Biomedical Image Segmentation. Evol. Comput. Comb. Optim. 2017, 10435, 399–407. [Google Scholar]
- Deep Active Learning for Axon-Myelin Segmentation on Histology Data; arXiv:1907.05143 [cs.CV]; Computer Vision and Pattern Recognition (cs.CV); Machine Learning (cs.LG); Cornell University: Ithaca, NY, USA, 2019.
- Daoud, B.; Morooka, K.; Kurazume, R.; Leila, F.; Mnejja, W.; Daoud, J. 3D segmentation of nasopharyngeal carcinoma from CT images using cascade deep learning. Comput. Med. Imaging Graph. 2019, 77, 101644. [Google Scholar] [CrossRef] [PubMed]
- Deep Learning for Automatic Tumour Segmentation in PET/CT Images of Patients with Head and Neck Cancers; arXiv:1908.00841 [eess.IV]; Image and Video Processing (eess.IV); Cornell University: Ithaca, NY, USA, 2019.
- Ma, Z.; Zhou, S.; Wu, X.; Zhang, H.; Yan, W.; Sun, S.; Zhou, J. Nasopharyngeal carcinoma segmentation based on enhanced convolutional neural networks using multi-modal metric learning. Phys. Med. Biol. 2018, 64, 025005. [Google Scholar] [CrossRef] [PubMed]
- Lentzen, M.-P.; Zirk, M.; Riekert, M.; Buller, J.; Kreppel, M. Anatomical and Volumetric Analysis of the Sphenoid Sinus by Semiautomatic Segmentation of Cone Beam Computed Tomography. J. Craniofacial Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Descoteaux, M.; Audette, M.A.; Chinzei, K.; Siddiqi, K. Bone enhancement filtering: Application to sinus bone segmentation and simulation of pituitary surgery. Comput. Aided Surg. 2006, 11, 247–255. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Training and Tuning (KUAH) (n = 83) | Internal-Validation (KUAH) (n = 20) | External-Validation KUANH (n = 20) |
|---|---|---|---|
| Age | 59.9 ± 17.2 | 63.1 ± 16.9 | 40 ± 19.7 |
| Male | 44 | 10 | 10 |
| Female | 39 | 10 | 10 |
| Tube voltage (kV) | 120 | 120 | 90 |
| Tube current (mA) | 5 | 5 | 4 |
| Scan time (s) | 16.8 | 16.8 | 14.3 |
| Voxel size (mm) | 0.3 | 0.3 | 0.3 |
| FOV (mm) | 230 × 170 | 230 × 170 | 170 × 135 |
| Focal spot (mm) | 0.58 | 0.58 | 0.70 |
| Mean ± SD (Range) | First Step | Second Step | Last Step |
|---|---|---|---|
| Air | 0.920 ± 0.17 (0.245–0.992) | 0.925 ± 0.16 (0.241–0.991) | 0.930 ± 0.16 (0.243–0.996) |
| Lesion | 0.770 ± 0.18 (0.208–0.912) | 0.750 ± 0.19 (0.205–0.975) | 0.760 ± 0.18 (0.208–0.96) |
| Mean ± SD (Range) | Last step (KUAH) | Last step (KUANH) |
|---|---|---|
| Air | 0.93 ± 0.16 (0.243–0.996) | 0.97 ± 0.02 (0.94–0.99) |
| Lesion | 0.76 ± 0.18 (0.208–0.96) | 0.54 ± 0.23 (0.12–0.88) |
| First Step | Second Step | Last Step | |
|---|---|---|---|
| Manual segmentation | CNN-assisted and manually modified segmentation | CNN-assisted and manually modified segmentation | |
| Time | 1824.0 s | 493.2 s | 362.7 s |
| Grade | Manual | 3D U-Net (Last Step for Active Learning) | ||||||
|---|---|---|---|---|---|---|---|---|
| KUAH | KUANH | KUAH | KUANH | |||||
| Air | Lesion | Air | Lesion | Air | Lesion | Air | Lesion | |
| 4—Very accurate | 75.7 | 75 | 83.7 | 79.7 | 91 | 90 | 95.3 | 88 |
| 3—Accurate | 19.6 | 16.6 | 15.3 | 19.3 | 8 | 7.4 | 4.7 | 12 |
| 2—Mostly accurate | 1 | 3.7 | 1 | 1 | 0 | 2.3 | 0 | 0 |
| 1—Inaccurate | 3.7 | 4.7 | 0 | 0 | 0 | 0.3 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-K.; Lim, H.-K.; Lee, S.; Cho, Y.; Song, I.-S. Deep Active Learning for Automatic Segmentation of Maxillary Sinus Lesions Using a Convolutional Neural Network. Diagnostics 2021, 11, 688. https://doi.org/10.3390/diagnostics11040688
Jung S-K, Lim H-K, Lee S, Cho Y, Song I-S. Deep Active Learning for Automatic Segmentation of Maxillary Sinus Lesions Using a Convolutional Neural Network. Diagnostics. 2021; 11(4):688. https://doi.org/10.3390/diagnostics11040688
Chicago/Turabian StyleJung, Seok-Ki, Ho-Kyung Lim, Seungjun Lee, Yongwon Cho, and In-Seok Song. 2021. "Deep Active Learning for Automatic Segmentation of Maxillary Sinus Lesions Using a Convolutional Neural Network" Diagnostics 11, no. 4: 688. https://doi.org/10.3390/diagnostics11040688
APA StyleJung, S.-K., Lim, H.-K., Lee, S., Cho, Y., & Song, I.-S. (2021). Deep Active Learning for Automatic Segmentation of Maxillary Sinus Lesions Using a Convolutional Neural Network. Diagnostics, 11(4), 688. https://doi.org/10.3390/diagnostics11040688








