68Ga-PSMA PET/CT for Patients with PSA Relapse after Radical Prostatectomy or External Beam Radiotherapy
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. 68Ga-PSMA PET/CT
2.3. Definitions
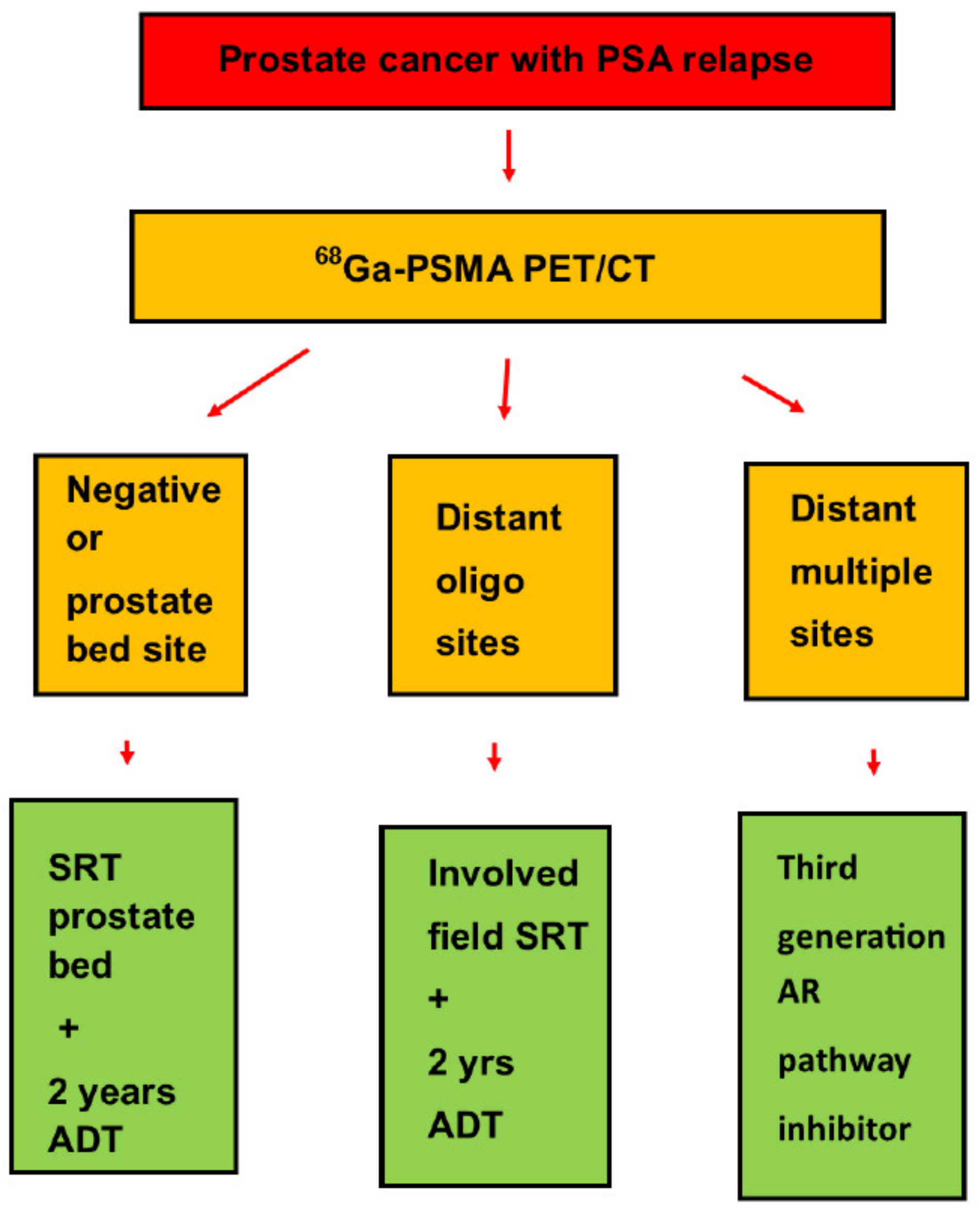
2.4. Salvage Treatment
2.5. Statistical Methods
3. Results
3.1. Initial Treatment
3.2. 68Ga-PSMA PET/CT
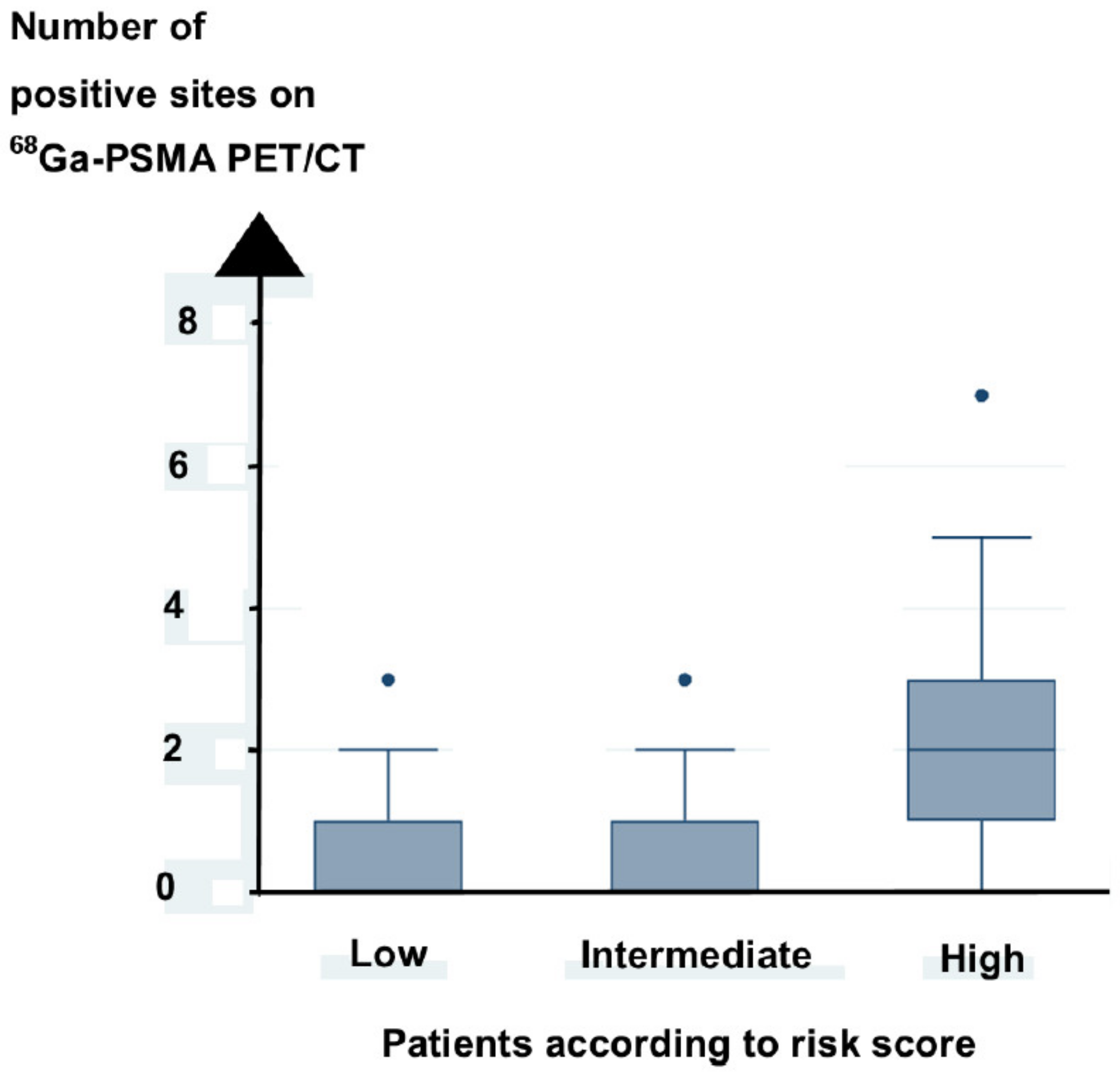
3.3. Prediction of Positive Sites on 68Ga-PSMA PET/CT
3.4. Salvage Treatment
3.5. Prediction of Overall Survival
4. Discussion
5. Conclusions
6. Ethical Approval
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonarakis, E.S.; Feng, Z.; Trock, B.J.; Humphreys, E.B.; Carducci, M.A.; Partin, A.W.; Walsh, P.C.; Eisenberger, M.A. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: Long-term follow-up. BJU Int. 2012, 109, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005, 294, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Antonarakis, E.S. Management of biochemically recurrent prostate cancer after local therapy: Evolving standards of care and new directions. Clin. Adv. Hematol. Oncol. 2013, 11, 14–23. [Google Scholar] [PubMed]
- Kestin, L.L.; Vicini, F.A.; Ziaja, E.L.; Stromberg, J.S.; Frazier, R.C.; Martinez, A.A. Defining biochemical cure for prostate carcinoma patients treated with external beam radiation therapy. Cancer 1999, 86, 1557–1566. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Moul, J.; Carroll, P.R.; Sun, L.; Lubeck, D.; Chen, M.H. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J. Urol. 2004, 172 Pt 2, S42–S46, discussion S46–S47. [Google Scholar] [CrossRef]
- Denham, J.W.; Steigler, A.; Wilcox, C.; Lamb, D.S.; Joseph, D.; Atkinson, C.; Matthews, J.; Tai, K.H.; Spry, N.A.; Christie, D.; et al. Time to biochemical failure and prostate-specific antigen doubling time as surrogates for prostate cancer-specific mortality: Evidence from the TROG 96.01 randomised controlled trial. Lancet Oncol. 2008, 9, 1058–1068. [Google Scholar] [CrossRef]
- Brockman, J.A.; Alanee, S.; Vickers, A.J.; Scardino, P.T.; Wood, D.P.; Kibel, A.S.; Lin, D.W.; Bianco, F.J., Jr.; Rabah, D.M.; Klein, E.A.; et al. Nomogram predicting prostate cancer-specific mortality for men with biochemical recurrence after radical prostatectomy. Eur. Urol. 2015, 67, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, Z.S.; Spratt, D.E.; Romesser, P.B.; Pei, X.; Zhang, Z.; Polkinghorn, W.; McBride, S.; Kollmeier, M.; Yamada, Y.; Zelefsky, M.J. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur. Urol. 2015, 67, 1009–1016. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C.; et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 197–209. [Google Scholar] [CrossRef]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.P.; Kubler, H.; Haberhorn, U.; Eisenhut, M.; et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after rtadical prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective comparison of 18F-Fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, P.J.; Stricker, P.; Hruby, G.; Kneebone, A.; Ting, F.; Thompson, B.; Nguyen, Q.; Ho, B.; Emmett, L. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016, 117, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Pfister, D.; Heidenreich, A.; Vogg, A.; Drude, N.I.; Voo, S.; Mottaghy, F.M.; Behrendt, F.F. Extent of disease in recurrent prostate cancer determined by [68Ga] PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 397–403. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Mier, W.; Haufe, S.; Debus, N.; Eder, M.; Eisenhut, M.; Schafer, M.; et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.T.; Radtke, J.P.; Afshar-Oromieh, A.; Roethke, M.C.; Hadaschik, B.A.; Gleave, M.; Bonekamp, D.; Kopka, K.; Eder, M.; Heusser, T.; et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: Comparison with mpMRI integrated in simultaneous PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 776–787. [Google Scholar] [CrossRef]
- Eissa, A.; Elsherbiny, A.; Coelho, R.F.; Rassweiler, J.; Davis, J.W.; Porpiglia, F.; Patel, V.R.; Prandini, N.; Micali, S.; Sighinolfi, M.C.; et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: A systematic review of the literature. Minerva Urol. Nefrol. 2018, 70, 462–478. [Google Scholar] [CrossRef]
- Boreta, L.; Gadzinski, A.J.; Wu, S.Y.; Xu, M.; Greene, K.; Quanstrom, K.; Nguyen, H.G.; Carroll, P.R.; Hope, T.A.; Feng, F.Y. Location of recurrence by Gallium-68 PSMA-11 PET scan in prostate cancer patients eligible for salvage radiotherapy. Urology 2019, 129, 165–171. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Buchholz, H.G.; Wieler, H.J.; Hofner, T.; Muller-Hubenthal, J.; Trampert, L.; Schreckenberger, M. The positivity rate of 68Gallium-PSMA-11 ligand PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Oncotarget 2019, 10, 6124–6137. [Google Scholar] [CrossRef]
- McCarthy, M.; Francis, R.; Tang, C.; Watts, J.; Campbell, A. A multicenter prospective clinical trial of 68Gallium PSMA HBED-CC PET-CT restaging in biochemically relapsed prostate carcinoma: Oligometastatic rate and distribution compared with standard imaging. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Schwenck, J.; Olthof, S.C.; Pfannenberg, C.; Reischl, G.; Wegener, D.; Marzec, J.; Bedke, J.; Stenzl, A.; Nikolaou, K.; la Fougere, C.; et al. Intention-to-treat analysis of 68Ga-PSMA and (11)C-Choline PET/CT versus CT for prostate cancer recurrence after surgery. J. Nucl. Med. 2019, 60, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Roberts, M.J.; Perera, M.; Williams, E.; Rhee, H.; Pryor, D.; Lehman, M.; Heathcote, P.; Wood, S.; Coucher, J.; et al. The clinical efficacy of PSMA PET/MRI in biochemically recurrent prostate cancer compared with standard of care imaging modalities and confirmatory histopathology: Results of a single-centre, prospective clinical trial. Clin. Exp. Metastasis 2020, 37, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.B.; Lima, E.N.P.; Zequi, S.C. Evaluation of the clinical use of PET/CT with 68Ga-PSMA for the assessment of biochemical recurrence of low or intermediate-risk prostate cancer. Urol. Oncol. 2021, 39, 73.e9–73.e18. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.R.; Yu, K.J.; Liu, F.Y.; Yang, L.Y.; Hong, J.H.; Yen, T.C.; Pang, S.T.; Wang, L.J. Comparison between 68Ga-PSMA-11 PET/CT and multiparametric magnetic resonance imaging in patients with biochemically recurrent prostate cancer following robot-assisted radical prostatectomy. J. Formos. Med. Assoc. 2021, 120, 688–696. [Google Scholar] [CrossRef]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective multicenter study of the diagnostic accuracy of prostate-specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY). J. Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.Y.; Wong, J.Y.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: Results from the CONDOR phase 3, multicenter study. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; da Cunha, M.L.; Wagner, J.; Haberkorn, U.; Debus, N.; Weber, W.; Eiber, M.; Holland-Letz, T.; Rauscher, I. Performance of [68Ga] Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer after prostatectomy-a multi-centre evaluation of 2533 patients. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- DAPROCA. [Imaging of Prostate Cancer v 2.0] (Billiediagnostik ved Prostatacancer Version 2.0). 2020, p. 1. Available online: www.daproca:billeddiagnostik_v2.0_pdf (accessed on 25 March 2021).
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: Treatment of relapsing and metastatic prostate cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Gandaglia, G.; Leni, R.; Fossati, N.; Cucchiara, V.; Montorsi, F.; Briganti, A. Prostate-specific membrane antigen imaging in clinical guidelines: European Association of Urology, National Comprehensive Cancer Network, and beyond. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef]
- FDA. FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer; U.S. Food & Drug Administration: Silver Springs, MD, USA.
- Cantiello, F.; Crocerossa, F.; Russo, G.I.; Gangemi, V.; Ferro, M.; Vartolomei, M.D.; Lucarelli, G.; Mirabelli, M.; Scafuro, C.; Ucciero, G.; et al. Comparison between 64Cu-PSMA-617 PET/CT and 18F-Choline PET/CT imaging in early diagnosis of prostate cancer biochemical recurrence. Clin. Genitourin. Cancer 2018, 16, 385–391. [Google Scholar] [CrossRef]
- Rauscher, I.; Duwel, C.; Haller, B.; Rischpler, C.; Heck, M.M.; Gschwend, J.E.; Schwaiger, M.; Maurer, T.; Eiber, M. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur. Urol. 2018, 73, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, E.N.; Tuncel, M.; Akyol, F.; Bilen, C.Y.; Baydar, D.E.; Karabulut, E.; Ozen, H.; Caglar, M. 68Ga-labelled PSMA ligand HBED-CC PET/CT imaging in patients with recurrent prostate cancer. World J. Urol. 2019, 37, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Bianchi, L.; Borghesi, M.; Schiavina, R.; Castellucci, P.; Ercolino, A.; Bianchi, F.M.; Barbaresi, U.; Polverari, G.; Brunocilla, E.; Fanti, S.; et al. Predictive accuracy and clinical benefit of a nomogram aimed to predict 68Ga-PSMA PET/CT positivity in patients with prostate cancer recurrence and PSA <1 ng/mL external validation on a single institution database. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2100–2105. [Google Scholar] [PubMed]
- Ceci, F.; Bianchi, L.; Borghesi, M.; Polverari, G.; Farolfi, A.; Briganti, A.; Schiavina, R.; Brunocilla, E.; Castellucci, P.; Fanti, S. Prediction nomogram for 68Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 136–146. [Google Scholar] [CrossRef]
- Luiting, H.B.; van Leeuwen, P.J.; Remmers, S.; Donswijk, M.; Busstra, M.B.; Bakker, I.L.; Brabander, T.; Stokkel, M.; van der Poel, H.G.; Roobol, M.J. Optimal timing of prostate specific membrane antigen positron emission tomography/computerized tomography for biochemical recurrence after radical prostatectomy. J. Urol. 2020, 204, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Hope, T.A.; Calais, J.; Fendler, W.P. Oliver Sartor Talks with Thomas A. Hope, Jeremie Calais, and Wolfgang P. Fendler about FDA approval of PSMA. J. Nucl. Med. 2021, 62, 146–148. [Google Scholar] [CrossRef]
- Fendler, W.P.; Ferdinandus, J.; Czernin, J.; Eiber, M.; Flavell, R.R.; Behr, S.C.; Wu, I.K.; Lawhn-Heath, C.; Pampaloni, M.H.; Reiter, R.E.; et al. Impact of 68Ga-PSMA-11 PET on the management of recurrent prostate cancer in a prospective single-arm clinical trial. J. Nucl. Med. 2020, 61, 1793–1799. [Google Scholar] [CrossRef]
- Sonni, I.; Eiber, M.; Fendler, W.P.; Alano, R.M.; Vangala, S.S.; Kishan, A.U.; Nickols, N.; Rettig, M.B.; Reiter, R.E.; Czernin, J.; et al. Impact of 68Ga-PSMA-11 PET/CT on staging and management of prostate cancer patients in various clinical settings: A prospective single-center Study. J. Nucl. Med. 2020, 61, 1153–1160. [Google Scholar] [CrossRef]
- Soydal, C.; Urun, Y.; Suer, E.; Nak, D.; Ozkan, E.; Kucuk, N.O. Predictor of 68Ga PSMA PET/CT positivity in patients with prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 226–230. [Google Scholar] [CrossRef]
- Grivas, N.; de Bruin, D.; Barwari, K.; van Muilekom, E.; Tillier, C.; van Leeuwen, P.J.; Wit, E.; Kroese, W.; van der Poel, H. Ultrasensitive prostate-specific antigen level as a predictor of biochemical progression after robot-assisted radical prostatectomy: Towards risk adapted follow-up. J. Clin. Lab. Anal. 2019, 33, e22693. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-targeted theranostic concept and first proof-of-concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Memorial Sloan Kettering Cancer Center. 2010. Available online: https://www.mskcc.org/nomograms/prostate/psa_doubling_time (accessed on 1 February 2018).
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, F.E.; Oprea-Lager, D.E.; Liu, Y.; Ost, P.; Bidaut, L.; Collette, L.; Deroose, C.M.; Goffin, K.; Herrmann, K.; Hoekstra, O.S.; et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: A consensus recommendation from the EORTC Imaging Group. Lancet Oncol. 2018, 19, e534–e545. [Google Scholar] [CrossRef]
- Francolini, G.; Detti, B.; Bottero, M.; Zilli, T.; Lancia, A.; Bruni, A.; Borghesi, S.; Mariotti, M.; Castellucci, P.; Fanti, S.; et al. Detection rate, pattern of relapse and influence on therapeutic decision of PSMA PET/CT in patients affected by biochemical recurrence after radical prostatectomy, a retrospective case series. Clin. Transl. Oncol. 2021, 23, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Hughes, S.; Mallia, A.; Gibson, V.; Young, J.; Aggarwal, A.; Morris, S.; Challacombe, B.; Popert, R.; Brown, C.; et al. The management impact of 68gallium-tris(hydroxypyridinone) prostate-specific membrane antigen (68Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 674–686. [Google Scholar] [CrossRef]
- Pereira Mestre, R.; Treglia, G.; Ferrari, M.; Pascale, M.; Mazzara, C.; Azinwi, N.C.; Llado, A.; Stathis, A.; Giovanella, L.; Roggero, E. Correlation between PSA kinetics and PSMA-PET in prostate cancer restaging: A meta-analysis. Eur. J. Clin. Investig. 2019, 49, e13063. [Google Scholar] [CrossRef]
- Bashir, U.; Tree, A.; Mayer, E.; Levine, D.; Parker, C.; Dearnaley, D.; Oyen, W.J.G. Impact of Ga-68-PSMA PET/CT on management in prostate cancer patients with very early biochemical recurrence after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, S.; Das, C.J.; Tripathi, M.; Seth, A.; Kumar, R.; Bal, C. Comparison of 68Ga-PSMA PET/CT and multiparametric MRI for staging of high-risk prostate cancer68Ga-PSMA PET and MRI in prostate cancer. Nucl. Med. Commun. 2017, 38, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; van Leeuwen, P.J.; Nandurkar, R.; Scheltema, M.J.; Cusick, T.; Hruby, G.; Kneebone, A.; Eade, T.; Fogarty, G.; Jagavkar, R.; et al. Treatment outcomes from 68Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: Prognostic value of a negative PSMA PET. J. Nucl. Med. 2017, 58, 1972–1976. [Google Scholar] [CrossRef]
- Celli, M.; De Giorgi, U.; Caroli, P.; Di Iorio, V.; Fantini, L.; Rossetti, V.; Foca, F.; Nicolini, S.; Giganti, M.; Paganelli, G.; et al. Clinical value of negative 68Ga-PSMA PET/CT in the management of biochemical recurrent prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 87–94. [Google Scholar] [CrossRef]
- Artibani, W.; Porcaro, A.B.; De Marco, V.; Cerruto, M.A.; Siracusano, S. Management of biochemical recurrence after primary curative treatment for prostate cancer: A review. Urol. Int. 2018, 100, 251–262. [Google Scholar] [CrossRef]
- Emmett, L.; Tang, R.; Nandurkar, R.; Hruby, G.; Roach, P.; Watts, J.A.; Cusick, T.; Kneebone, A.; Ho, B.; Chan, L.; et al. 3-year freedom from progression after 68Ga-PSMA PET/CT-triaged management in men with biochemical recurrence after radical prostatectomy: Results of a prospective multicenter trial. J. Nucl. Med. 2020, 61, 866–872. [Google Scholar] [CrossRef]
- Stish, B.J.; Pisansky, T.M.; Harmsen, W.S.; Davis, B.J.; Tzou, K.S.; Choo, R.; Buskirk, S.J. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J. Clin. Oncol. 2016, 34, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: Results of an australian prospective multicenter study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; De Phung, B.S.; et al. Enzalutamide in men with nonmetastatic castration-resistant prostate cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, C.; Wei, Y.; Meng, J.; Zhang, Y.; Gan, H.; Xu, X.; Wan, F.; Pan, J.; Ma, X.; et al. A Prospective trial of 68Ga-PSMA and 18F-FDG PET/CT in nonmetastatic prostate cancer patients with an early PSA progression during castration. Clin. Cancer Res. 2020, 26, 4551–4558. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE Phase 2 randomized clinical trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Kneebone, A.; Hruby, G.; Ainsworth, H.; Byrne, K.; Brown, C.; Guo, L.; Guminski, A.; Eade, T. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur. Urol. Oncol. 2018, 1, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef]
- Jansen, B.H.E.; Bodar, Y.J.L.; Zwezerijnen, G.J.C.; Meijer, D.; van der Voorn, J.P.; Nieuwenhuijzen, J.A.; Wondergem, M.; Roeleveld, T.A.; Boellaard, R.; Hoekstra, O.S.; et al. Pelvic lymph-node staging with 18F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer—The SALT trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 509–520. [Google Scholar] [CrossRef]
- Kopp, J.; Kopp, D.; Bernhardt, E.; Manka, L.; Beck, A.; Gerullis, H.; Karakiewicz, P.; Schoerner, W.; Hammerer, P.; Schiffmann, J. 68Ga-PSMA PET/CT based primary staging and histological correlation after extended pelvic lymph node dissection at radical prostatectomy. World J. Urol. 2020, 38, 3085–3090. [Google Scholar] [CrossRef]
- Calais, J.; Czernin, J.; Fendler, W.P.; Elashoff, D.; Nickols, N.G. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer 2019, 19, 18. [Google Scholar]
- Rousseau, E.; Wilson, D.; Lacroix-Poisson, F.; Krauze, A.; Chi, K.; Gleave, M.; McKenzie, M.; Tyldesley, S.; Goldenberg, S.L.; Benard, F. A prospective study on 18F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate cancer. J. Nucl. Med. 2019, 60, 1587–1593. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Cassarino, G.; Artioli, P.; Cecchin, D.; Dal Moro, F.; Zucchetta, P. PET/MRI in prostate cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]


| Patient Characteristic | No pts | MedianValue | Lower and Upper IQ | Total Range | |
|---|---|---|---|---|---|
| 95 | |||||
| Age (years) | 68 | 64–72 | 55–87 | ||
| Initial TNM staging | T1 | 6 | |||
| T2 | 53 | ||||
| T3 | 18 | ||||
| N0 | 72 | ||||
| N1 | 23 | ||||
| ISUP score | 1 | 9 | |||
| 2–3 | 32 | ||||
| 4 | 27 | ||||
| 5 | 26 | ||||
| Risk | Low | 11 | |||
| Intermediate | 10 | ||||
| High | 54 | ||||
| Initial treatment | RP | 45 | |||
| RP and adjuvant ADT | 49 | ||||
| RP and adjuvant EBRT | 14 | ||||
| EBRT | |||||
| Relapse-free interval until PSA relapse (mos) | 24 | 13–40 | 4–156 | ||
| PSADT (mos) | 10.6 | 4.5–48 | 1–168 | ||
| Time from the latest PSA before the 68Ga-PSMA PET/CT to the scan | 15 | 11–19 | 6–27 | ||
| PSA at 68Ga-PSMA PET/CT (ng/mL) | 5.7 | 1.2–12.6 | 0.10–155.6 | ||
| 68Ga-PSMA PET/CT sites | negative | 35 | |||
| Prostate bed | 12 | ||||
| Regional lymph nodes | 29 | ||||
| Distant organs | 19 | ||||
| Number of positive sites on 68Ga-PSMA PET/CT | 0 | 35 | |||
| 1 | 24 | ||||
| 2 | 13 | ||||
| 3 | 12 | ||||
| 4 | 5 | ||||
| 5 | 3 | ||||
| 7 | 2 | ||||
| Treatment after the 68Ga-PSMA PET/CT | Active surveillance | 7 | |||
| Salvage radiotherapy | 36 | ||||
| Docetaxel | 31 | ||||
| Abiraterone/Enzalutamide | 21 |
| Clinical Characteristics | Prediction in Logistic Regression Analysis | |
|---|---|---|
| Univariate analysis | Multivariate analysis | |
| Age | NS | NS |
| N status | NS | NS |
| ISUP score | 0.005 | NS |
| Risk score | <0.005 | NS |
| Number of positive sites | NS | NS |
| Initial treatment (RP vs. EBRT) | NS | NS |
| Adjuvant ADT | 0.002 | NS |
| Disease free interval | NS | NS |
| Lymph node metastases at initial treatment | NS | NS |
| PSADT | <0.0005 | <0.0005 |
| PSA at 68Ga-PSMA PET/CT | 0.010 | NS |
| Predictive Factors | Cox Regression Analysis | |
|---|---|---|
| Univariate | Multiple | |
| ISUP score | NS | - |
| Risk score | 0.039 | NS |
| Initial treatment | NS | - |
| Adjuvant ADT | NS | - |
| Interval from initial treatment to PSA recurrence | NS | - |
| PSA at 68Ga-PSMA PET/CT | NS | - |
| PSADT | NS | - |
| Most advanced regional location at 68Ga PSMA PET/CT | 0.004 | NS |
| No of sites on 68Ga-PSMA PET/CT | 0.0001 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Eyben, F.E.; Soydal, C.; von Eyben, R. 68Ga-PSMA PET/CT for Patients with PSA Relapse after Radical Prostatectomy or External Beam Radiotherapy. Diagnostics 2021, 11, 622. https://doi.org/10.3390/diagnostics11040622
von Eyben FE, Soydal C, von Eyben R. 68Ga-PSMA PET/CT for Patients with PSA Relapse after Radical Prostatectomy or External Beam Radiotherapy. Diagnostics. 2021; 11(4):622. https://doi.org/10.3390/diagnostics11040622
Chicago/Turabian Stylevon Eyben, Finn Edler, Cigdem Soydal, and Rie von Eyben. 2021. "68Ga-PSMA PET/CT for Patients with PSA Relapse after Radical Prostatectomy or External Beam Radiotherapy" Diagnostics 11, no. 4: 622. https://doi.org/10.3390/diagnostics11040622
APA Stylevon Eyben, F. E., Soydal, C., & von Eyben, R. (2021). 68Ga-PSMA PET/CT for Patients with PSA Relapse after Radical Prostatectomy or External Beam Radiotherapy. Diagnostics, 11(4), 622. https://doi.org/10.3390/diagnostics11040622






