Efficacy and Haematologic Toxicity of Palliative Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with Lutetium-177-Labeled Prostate-Specific Membrane Antigen in Heavily Pre-Treated Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment and Response Assessment
2.3. Haematologic Toxicity and Survival Analysis
3. Results
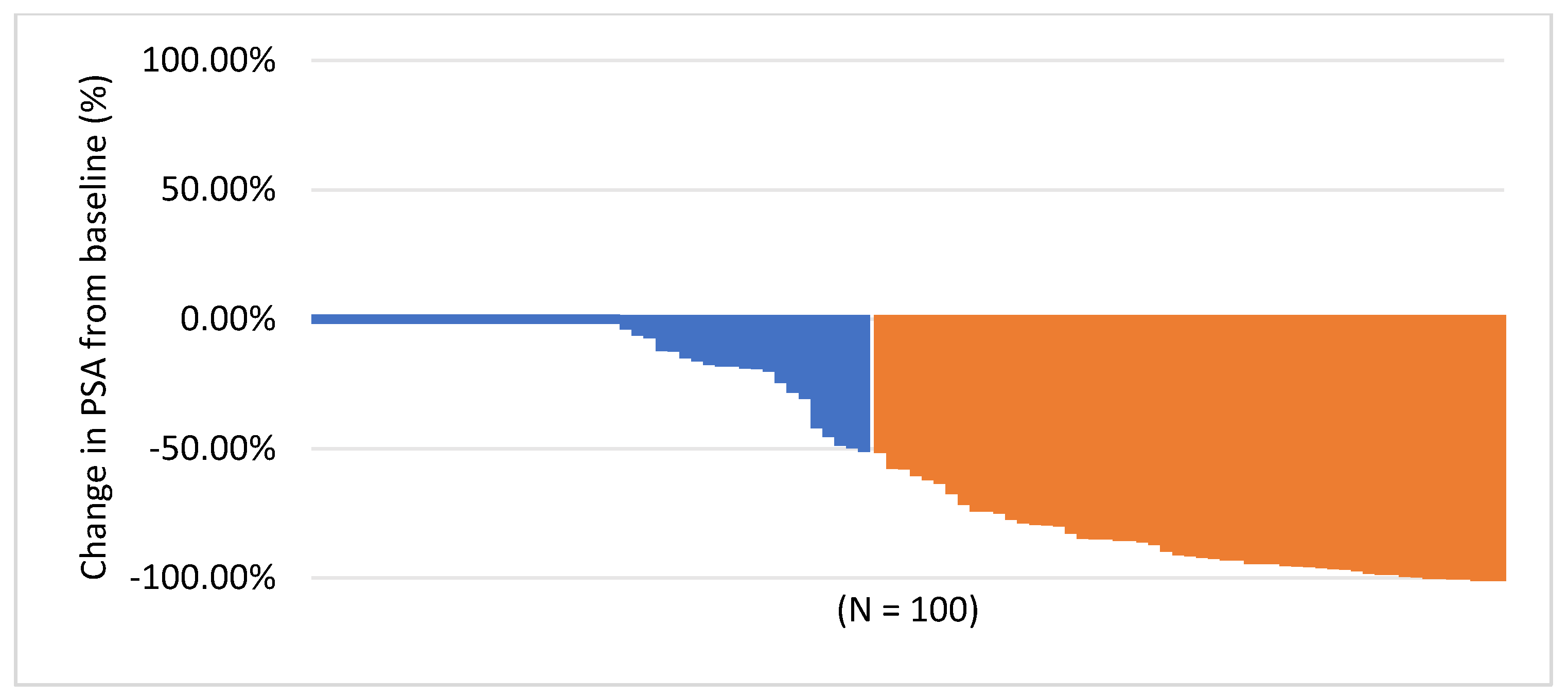
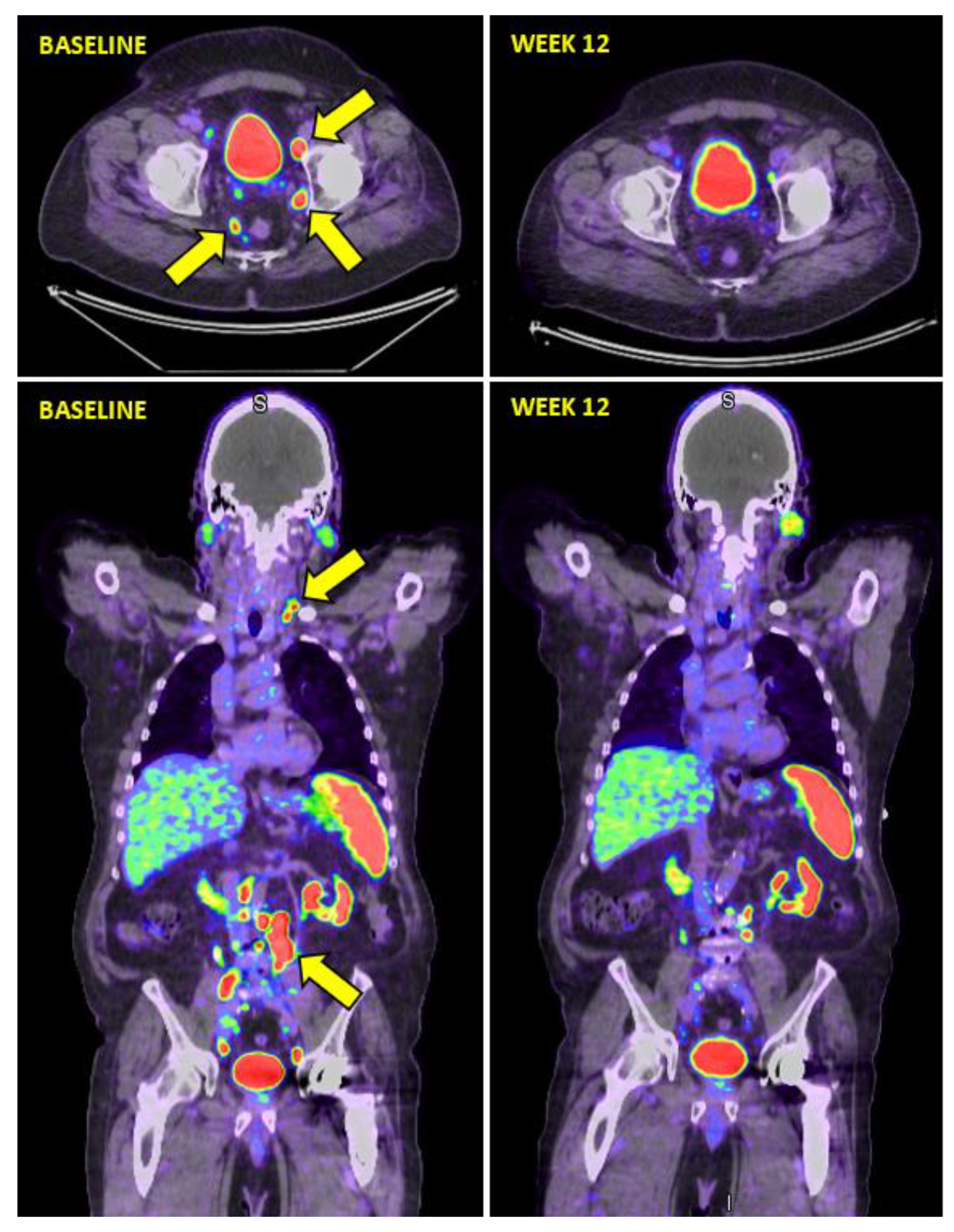
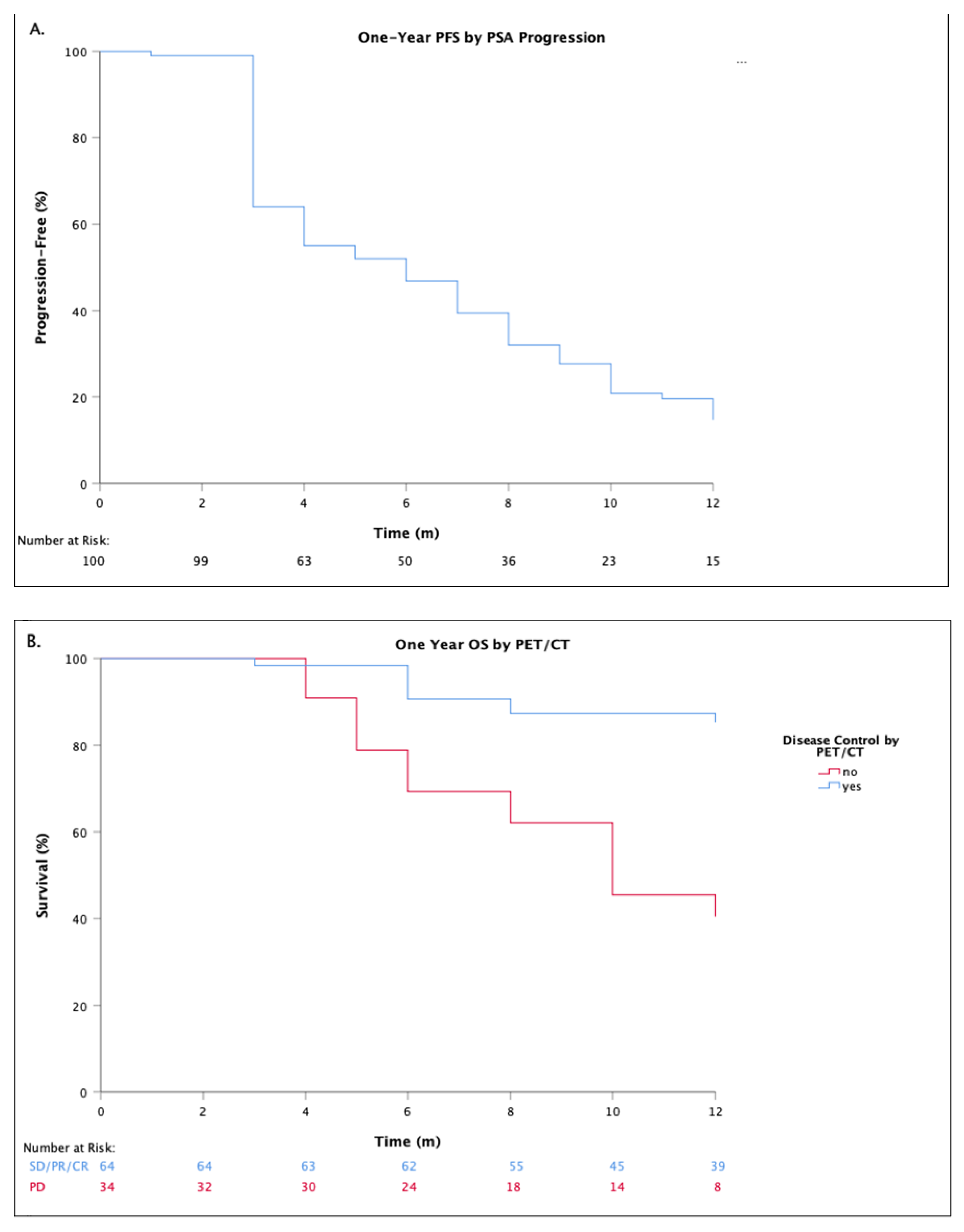
3.1. Response Assessment
3.2. Haematologic Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 68Ga-PSMA-11 PET/CT | Gallium-68-PSMA-11 positron emission tomography/computed tomography |
| 177Lu | Lutetium-177 |
| 177Lu-PSMA I&T | 177Lu-DOTAGA-(I-y)fk(Sub-KuE) |
| ADT | Androgen deprivation therapy |
| AL | Acute leukaemia |
| EANM | European Association of Nuclear Medicine |
| EAU | European Association of Urology |
| EBRT | External beam radiotherapy |
| ECOG | European Cooperative Oncology Group |
| IRB | institutional review board |
| NCI-CTCAE | National Cancer Institute Common Terminology Criteria for Adverse Events |
| mCRPC | Metastatic castrate resistant prostate cancer |
| MDS | Myelodysplastic syndrome |
| OS | Overall survival |
| OR | odds ratio |
| PERCIST | PET response criteria in solid tumors |
| PET/CT | Gallium-68-PSMA-11 positron emission tomography/computed tomography |
| PCWG | Prostate Cancer Working Group |
| PFS | Progression-free survival |
| PSA | Prostate-specific antigen |
| PSMA | Prostate-specific membrane antigen |
| RLT | Radioligand therapy |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Penson, D.F.; Armstrong, A.J.; Concepcion, R.; Agarwal, N.; Olsson, C.; Karsh, L.; Dunshee, C.; Wang, F.; Wu, K.; Krivoshik, A.; et al. Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J. Clin Oncol. 2016, 34, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Tombal, B.; Saad, F.; Penson, D.; Hussain, M.; Sternberg, C.N.; Morlock, R.; Ramaswamy, K.; Ivanescu, C.; Attard, G. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 556–569. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.K.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. TheraP: A randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603). J. Clin. Oncol. 2020, 38, 5500. [Google Scholar] [CrossRef]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with (177)Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.S.; Halabi, S.; Smith, M.R.; Basch, E.M.; Fizazi, K.; Ryan, C.J.; Antonarakis, E.S.; et al. The Prostate Cancer Working Group 3 (PCWG3) consensus for trials in castration-resistant prostate cancer (CRPC). J. Clin. Oncol. 2015, 33, 5000. [Google Scholar] [CrossRef]
- Kesavan, M.; Turner, J.H.; Meyrick, D.; Yeo, S.; Cardaci, G.; Lenzo, N.P. Salvage Radiopeptide Therapy of Advanced Castrate-Resistant Prostate Cancer with Lutetium-177-Labeled Prostate-Specific Membrane Antigen: Efficacy and Safety in Routine Practice. Cancer Biother. Radiopharm. 2018, 33, 274–281. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with (177)Lu-labelled PSMA-ligands ((177)Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Grubmuller, B.; Senn, D.; Kramer, G.; Baltzer, P.; D’Andrea, D.; Grubmuller, K.H.; Mitterhauser, M.; Eidherr, H.; Haug, A.R.; Wadsak, W.; et al. Response assessment using (68)Ga-PSMA ligand PET in patients undergoing (177)Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1063–1072. [Google Scholar] [CrossRef]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouviere, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA Theranostics: Review of the Current Status of PSMA-Targeted Imaging and Radioligand Therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Bauman, G.; von Eyben, R.; Rahbar, K.; Soydal, C.; Haug, A.R.; Virgolini, I.; Kulkarni, H.; Baum, R.; Paganelli, G. Optimizing PSMA Radioligand Therapy for Patients with Metastatic Castration-Resistant Prostate Cancer. A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 9054. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Kessel, K.; Schlack, K.; Weckesser, M.; Bogemann, M.; Rahbar, K. Radioligand therapy using [(177)Lu]Lu-PSMA-617 in mCRPC: A pre-VISION single-center analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2106–2112. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events: (CTCAE); NCI Cancer Therapy Evaluation Program: Bethesda, MD, USA, 2010. [Google Scholar]
- Sartor, A.O.; Morris, M.J.; Messman, R.; Krause, B.J. VISION: An international, prospective, open-label, multicenter, randomized phase III study of 177Lu-PSMA-617 in the treatment of patients with progressive PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, TPS259. [Google Scholar] [CrossRef]
- Grochtdreis, T.; Konig, H.H.; Dobruschkin, A.; von Amsberg, G.; Dams, J. Cost-effectiveness analyses and cost analyses in castration-resistant prostate cancer: A systematic review. PLoS ONE 2018, 13, e0208063. [Google Scholar] [CrossRef]
- Bryce, A.H.; Alumkal, J.J.; Armstrong, A.; Higano, C.S.; Iversen, P.; Sternberg, C.N.; Rathkopf, D.; Loriot, Y.; de Bono, J.; Tombal, B.; et al. Radiographic progression with nonrising PSA in metastatic castration-resistant prostate cancer: Post hoc analysis of PREVAIL. Prostate Cancer Prostatic Dis. 2017, 20, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The Impact of (68)Ga-PSMA PET/CT on Management Intent in Prostate Cancer: Results of an Australian Prospective Multicenter Study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef] [PubMed]
| n = 100 (%) | |
|---|---|
| Age (Years): Median (range) | 70 (range 50–89) |
| Age (Years) ≥ 70 | 54 (54) |
| PSA (ng/mL): Median (range) | 73 (range 0.1–5000) |
| Median Gleason Score | 9 (range 6–10) |
| Metastases | 100 (100) |
| Nodal | 12 (12) |
| Bone (>15 lesions) | 26 (26) |
| Nodal + Bone | 61 (61) |
| Other | 1 (1) |
| Number of Lines of Prior Therapy | |
| 1 | 1 (1) |
| 2 | 16 (16) |
| 3 | 34 (34) |
| 4 | 28 (28) |
| 5 | 18 (18) |
| 6 | 3 (3) |
| Primary Therapy | |
| Radical prostatectomy | 42 (42) |
| ADT | 23 (23) |
| EBRT | 26 (26) |
| Chemotherapy + ADT | 9 (9) |
| Prior Hormone Therapies * | |
| Abiraterone | 40 (40) |
| Bicalutamide | 36 (36) |
| Enzalutamide | 48 (48) |
| Apalutamide | 1 (1) |
| Nilutamide | 1 (1) |
| Prior Chemotherapy | |
| Docetaxel | 29 (29) |
| Cabazitaxel | 5 (5) |
| Prior Docetaxel and Cabazitaxel | 20 (20) |
| Mitoxantrone | 3 (3) |
| Other Prior Therapies | |
| Palliative radiotherapy | 65 (65) |
| Radium | 4 (4) |
| PRRT | 2 (2) |
| Concurrent Hormone Therapy | 14(14) |
| Abiraterone | 6 (6) |
| Enzalutamide | 6 (6) |
| Bicalutamide | 1 (1) |
| Other | 1 (1) |
| Number of Cycles Completed | |
| 1 | 1(1) |
| 2 | 56 (43) |
| 3 | 27 (27) |
| 4 | 15 (15) |
| 5 | 1 (1) |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Univariable Analyses | |||
| Age ≥ 70 Years | 1.47 | 0.42–5.26 | 0.55 |
| Prior Palliative Radiotherapy (skeletal) | 0.38 | 0.08–1.85 | 0.21 |
| Prior Chemotherapy | 1.18 | 0.32–4.46 | 0.78 |
| Prior Docetaxel and Cabazitaxel | 0.63 | 0.15–2.62 | 0.69 |
| Presence of Bone and Nodal Metastases | 1.34 | 0.38–4.76 | 0.64 |
| 177Lu-PSMA I&T Cycles Completed | |||
| 1 | ** | ||
| 2 | 0.70 | 0.19–2.56 | 0.59 |
| 3 | 0.61 | 0.16–2.27 | 0.46 |
| 4 | ** | ||
| Multivariable Analyses | |||
| Age ≥ 70 years | 1.68 | 0.43–6.53 | 0.45 |
| Prior Chemotherapy | 1.18 | 0.28–4.96 | 0.82 |
| Prior Docetaxel and Cabazitaxel | 0.61 | 0.10–3.54 | 0.57 |
| Presence of Bone and Nodal Metastases | 1.35 | 0.36–5.14 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesavan, M.; Meyrick, D.; Gallyamov, M.; Turner, J.H.; Yeo, S.; Cardaci, G.; Lenzo, N.P. Efficacy and Haematologic Toxicity of Palliative Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with Lutetium-177-Labeled Prostate-Specific Membrane Antigen in Heavily Pre-Treated Patients. Diagnostics 2021, 11, 515. https://doi.org/10.3390/diagnostics11030515
Kesavan M, Meyrick D, Gallyamov M, Turner JH, Yeo S, Cardaci G, Lenzo NP. Efficacy and Haematologic Toxicity of Palliative Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with Lutetium-177-Labeled Prostate-Specific Membrane Antigen in Heavily Pre-Treated Patients. Diagnostics. 2021; 11(3):515. https://doi.org/10.3390/diagnostics11030515
Chicago/Turabian StyleKesavan, Murali, Danielle Meyrick, Marat Gallyamov, J. Harvey Turner, Sharon Yeo, Giuseppe Cardaci, and Nat P. Lenzo. 2021. "Efficacy and Haematologic Toxicity of Palliative Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with Lutetium-177-Labeled Prostate-Specific Membrane Antigen in Heavily Pre-Treated Patients" Diagnostics 11, no. 3: 515. https://doi.org/10.3390/diagnostics11030515
APA StyleKesavan, M., Meyrick, D., Gallyamov, M., Turner, J. H., Yeo, S., Cardaci, G., & Lenzo, N. P. (2021). Efficacy and Haematologic Toxicity of Palliative Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with Lutetium-177-Labeled Prostate-Specific Membrane Antigen in Heavily Pre-Treated Patients. Diagnostics, 11(3), 515. https://doi.org/10.3390/diagnostics11030515






