Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Reliability Study in Axial Spondyloarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Eligibility and Recruitment
2.3. Data Collection and Baseline Assessments
2.4. Assessment Schedule
2.5. Data Management
2.5.1. Sensor Data Output
2.5.2. Calculation of Composite Metrology Score—IMU-ASMI (Amb)
2.6. Statistical Methods
3. Results
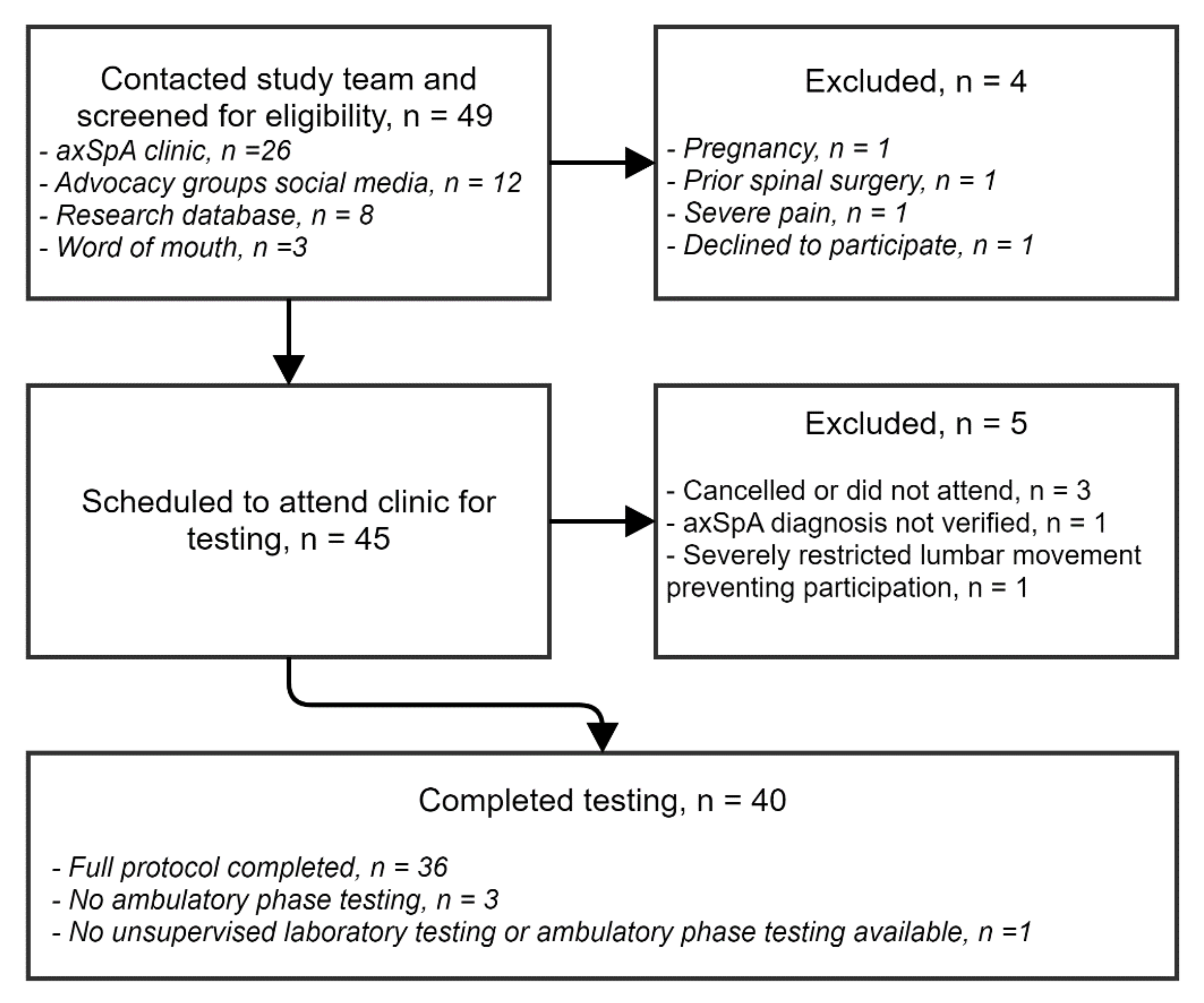
3.1. Recruitment and Participant Characteristics
3.2. Protocol Fidelity
3.3. Spinal Mobility Data
3.4. Reliability and Agreement of IMU Movements
3.5. Reliability and Agreement of IMU-ASMI (Amb) Indices
3.6. Pain and Fatigue Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Baraliakos, X.; Heldmann, F.; Callhoff, J.; Listing, J.; Appelboom, T.; Brandt, J.; Van den Bosch, F.; Breban, M.; Burmester, G.; Dougados, M.; et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observation study using MRI and conventional radiography. Ann. Rheum. Dis. 2014, 73, 1819–1825. [Google Scholar] [CrossRef]
- Wanders, A.; Landewé, R.; Dougados, M.; Mielants, H.; Van der Linden, S.; Van der Heijde, D. Association between radiographic damage of the spine and spinal mobility for individuals with ankylosing spondylitis: Can assessment of spinal mobility be a proxy for radiographic evaluation? Ann. Rheum. Dis. 2005, 64, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijde, D.; Ramiro, S.; Landewé, R.; Baraliakos, X.; Van den Bosch, F.; Sepriano, A.; Regel, A.; Ciurea, A.; Dagfinrud, H.; Dougados, M.; et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 978–991. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Calin, A.; Dougados, M.; Khan, M.; Van der Linden, S.; Bellamy, N. Selection of instruments in the core set for DC-ART, SMARD, physical therapy, and clinical record keeping in ankylosing spondylitis. Progress report of the ASAS Working Group. Assessments in Ankylosing Spondylitis. J. Rheumatol. 1999, 26, 951–954. [Google Scholar]
- Sieper, J.; Rudwaleit, M.; Baraliakos, X.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Dougados, M.; Hermann, K.G.; Landewé, R.; van der Heijde, D. The Assessment of SpondyloArthritis international Society (ASAS) handbook: A guide to assess spondyloarthritis. Ann. Rheum. Dis. 2009, 68, ii1–ii44. [Google Scholar] [CrossRef]
- Landewé, R.; Van Tubergen, A. Clinical Tools to Assess and Monitor Spondyloarthritis. Curr. Rheumatol. Rep. 2015, 17, 522. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, T.; Mallorie, P.; Whitelock, H.; Kennedy, L.; Garrett, S.; Calin, A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J. Rheumatol. 1994, 21, 1694–1698. [Google Scholar]
- Jauregui, E.; Conner-Spady, B.; Russel, A.; Maksymowych, W. Clinimetric evaluation of the bath ankylosing spondylitis metrology index in a controlled trial of pamidronate therapy. J. Rheumatol. 2004, 31, 2422–2428. [Google Scholar]
- Auleley, G.; Benbouazza, K.; Spoorenberg, A.; Collantes, E.; Hajja-Hassouni, N.; Van der Heijde, D.; Dougados, M. Evaluation of the smallest detectable difference in outcome or process variables in ankylosing spondylitis. Arthrit. Care Res. 2002, 47, 582–587. [Google Scholar] [CrossRef]
- Calvo-Gutiérrez, J.; Garrido-Castro, J.; Conzález-Navas, C.; Castro-Villegas, C.; Ortega-Castro, R.; López-Medina, C.; Font-Ugalde, P.; Escudero-Contreras, A.; Collantes-Estévez, E. Inter-rater reliability of clinical mobility measures in ankylosing spondylitis. BMC Musculoskelet Disord. 2016, 17, 382. [Google Scholar] [CrossRef]
- American College of Rheumatology. Position statement of Telemedicine. 29 June 2020. Available online: https://www.rheumatology.org/Portals/0/Files/Telemedicine-Position-Statement.pdf (accessed on 7 November 2020).
- Papi, E.; Koh, W.; McGregor, A. Wearable technology for spine movement assessment: A systematic review. J. Biomech. 2017, 64, 186–197. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.; Galán-Mercant, A.; Williams, J. The use of inertial sensors system for human motion analysis. Phys. Ther. Rev. 2010, 15, 462–473. [Google Scholar] [CrossRef]
- Simpson, L.; Maharaj, M.; Mobbs, R. The role of wearables in spinal posture analysis: A systematic review. BMC Musculoskelet Disord. 2019, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; Lech, M.; Taylor, N.; Cosic, I. A reliability study of the new back strain monitor based on clinical trials. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 693–696. [Google Scholar]
- Charry, E.; Umer, M.; Taylor, S. Design and validation of an ambulatory inertial system for 3-D measurements of low back movements. In Proceedings of the 2011 Seventh International Conference on Intelligent Sensors, Sensor Networks and Information Processing, Adelaide, SA, Australia, 6–9 December 2011; pp. 58–63. [Google Scholar]
- Aranda-Valera, I.; Cuesta-Vargas, A.; Garrido-Castro, J.; Gardiner, P.; López-Medina, C.; Machado, P.; Condell, J.; Connolly, J.; Williams, J.; Muñoz-Esquivel, K.; et al. Measuring spinal mobility using an inertial measurement unity system: A validation study in axial spondyloarthritis. Diagnostics 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, P.; Small, D.; Muñoz-Esquivel, K.; Condell, J.; Cuesta-Vargas, A.; Williams, J.; Machado, P.; Garrido-Castro, J. Validity and reliability of a sensor-based electronic spinal mobility index for axial spondyloarthritis. Rheumatology 2020, 59, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Jenkinson, T.; Kennedy, G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis disease activity index. J. Rheumatol. 1994, 1, 2286–2291. [Google Scholar]
- Calin, A.; Garett, S.; Whitelock, H.; Kennedy, L.; O’Hea, J.; Mallorie, P.; Jenkinson, T. A new approach to defining functional ability in ankylosing spondylitis: The development of the Bath Ankylosing Spondylitis Functional Index. J. Rheumatol. 1994, 21, 2281–2285. [Google Scholar]
- Jones, S.; Steiner, A.; Garrett, S.; Calin, A. The bath ankylosing spondylitis patient global score (BAS-G). Rheumatology 1996, 35, 66–71. [Google Scholar] [CrossRef]
- Mjøsund, H.L.; Boyle, E.; Kjaer, P.; Mieritz, R.M.; Skallgård, T.; Kent, P. Clinically acceptable agreement between the ViMove wireless motion sensor system and the Vicon motion capture system when measuring lumbar region inclination motion in the sagittal and coronal planes. BMC Musculoskelet Disord. 2017, 18, 124. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Landewé, R.; Feldkeller, E. Proposal of a linear definition of the Bath Ankylosing Spondylitis Metrology Index (BASMI) and comparison with the 2-step and 10-step definitions. Ann. Rheum. Dis. 2008, 67, 489–493. [Google Scholar] [CrossRef]
- Spies, C.M.; Cutolo, M.; Straub, R.H.; Burmester, G.-R.; Buttgereit, F. More night than day—Circadian rhythms in polymyalgia rheumatica and ankylosing spondylitis. J. Rheumatol. 2010, 37, 894–899. [Google Scholar] [CrossRef]
- Koo, T.; Li, M. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.H.; Luk, K.D.; Leong, J.C.; Woo, C.W. Torsional stability of the lumbosacral junction. Significance of the iliolumbar ligament. Spine 1989, 14, 611–615. [Google Scholar] [CrossRef]
- White, A.A.; Panjabi, M.M. Clinical Biomechanics of the Spine, 2nd ed.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 1990. [Google Scholar]
- Bogduk, N. Functional anatomyof the spina. Handb. Clin. Neurol. 2016, 136, 675–688. [Google Scholar]
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014, 67, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, L.; Zanframundo, G.; Codullo, V.; Grazia Pisu, M.; Caporali, R.; Montecucco, C. Telemedicine in rheumatology: A reliable approach beyond the pandemic. Rheumatology 2020. [Google Scholar] [CrossRef]
- Opinc, A.; Lukasik, Z.; Makowska, J. The attitude of Polish rheumatology patients towards telemedicine in the age of the COVID-19 pandemic. Reumatologia 2020, 58, 134–141. [Google Scholar] [CrossRef]
- López-Medina, C.; Escudero, A.; Collantes-Estevez, E. COVID-19 pandemic: An opportunity to assess the utility of telemedicine in patients with rheumatic diseases. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Devadula, S.; Langbecker, D.; Vecchio, P.; Meiklejohn, J.; Benham, H. Tele-rheumatology to regional hospital outpatient clinics. Patient perspectives on a new model of care. Telemed J. E-Health 2020, 26. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Green Paper on Mobile Health (“mHealth”). 2014. Available online: https://ec.europa.eu/digital-single-market/en/news/green-paper-mobile-health-mhealth (accessed on 7 November 2020).
- Magnol, M.; Berard, E.; Rempenault, C.; Castagne, B.; Pugibet, M.; Lukas, C.; Tournadre, A.; Vergne-Salle, P.; Barnetche, T.; Truchetet, M.E.; et al. Use of eHealth by patients with rheumatoid arthritis: An observational, cross sectional, multicenter study. Ann. Rheum. Dis. 2020, 79, 532–533. [Google Scholar] [CrossRef]
- Knitza, J.; Simon, D.; Lambrecht, A.; Raab, C.; Tascilar, K.; Hagen, M.; Kleyer, A.; Bayat, S.; Derungs, A.; Amft, O.; et al. Mobile health usage, preferences, barriers, and eHealth literacy in Rheumatology: Patient survey study. JMIT Mhealth Uhealth 2020, 8, 19661. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, P.; Lu, F.; Elias, J.; Sparks, J.A.; Lee, Y.C. Mobile apps for individuals with rheumatoid arthritis: A systematic review. J. Clin. Rheumatol. 2019, 25, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Grainger, R.; Townsley, H.; White, B.; Langlotz, T.; Taylor, W.J. Apps for people with rheumatoid arthritis to monitor their disease activity: A review of apps for best practice and quality. JMIR Mhealth Uhealth 2017, 5, e7. [Google Scholar] [CrossRef]
- Najm, A.; Gossec, L.; Weill, C.; Benoist, D.; Berenbaum, F.; Nikiphorou, E. Mobile Health Apps for Self-Management of Rheumatic and Musculoskeletal Diseases: Systematic Literature Review. JMIR mHealth uHealth 2019, 7, e14730. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Gasparini, S.; Ciapetti, A.; Gutierrez, M.; Grassi, W. Usability of an innovative and interactive electronic system for collection of patient-reported data in axial spondyloarthritis: Comparison with the traditional paper-administered format. Rheumatology 2013, 52, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.R.P.; de Sousa, H.M.L.; Monteiro, J.A.F.; Lima, A.R.N.P. Future perspectives of smartphone applications for rheumatic diseases self-management. Rheumatol. Int. 2015, 35, 419–431. [Google Scholar] [CrossRef]
- Van Der Veer, S.; Austin, L.; Sanders, C.; Dixon, W. Using smartphones to improve remote monitoring of rheumatoid arthritis: Completeness of patients’ symptom reports. Ann. Rheum. Dis. 2017, 76, 547. [Google Scholar]
- Barlow, J.; Wright, C.; Sheasby, J.; Turner, A.; Hainsworth, J. Self-management approaches for people with chronic conditions: A review. Patient Educ. Couns. 2002, 48, 177–187. [Google Scholar] [CrossRef]
- Risling, T.; Martinez, J.; Young, J.; Thorp-Froslie, N. Evaluating patient empowerment in association with eHealth technology: Scoping review. J. Med. Internet Res. 2017, 19, e329. [Google Scholar] [CrossRef]
- Bradway, M.; Årsand, E.; Grøttland, A. Mobile health: Empowering patients and driving change. Trends Endocrinol. Metab. 2015, 26, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.M.; Stanescu, S.; Bishop, F.L. The use of measurement systems to support patient self-management of long-term conditions: An overview of opportunities and challenges. Patient Relat. Outcome Meas. 2019, 10, 385–394. [Google Scholar] [CrossRef] [PubMed]

| Day 1—Laboratory | Ambulatory Phase | Day 2—Laboratory | |
|---|---|---|---|
| Baseline data collection | √ | — | — |
| BASMILin and chest expansion | √ | — | √ |
| Pain NRS and Fatigue NRS | √ | √ | √ |
| Spinal movement tests | Supervised and Unsupervised | Unsupervised | Supervised and Unsupervised |
| Variable | AxSpA Cohort (n = 40) |
|---|---|
| Age, years | 48.0 (12.9); [27–76] |
| Sex (male/female), n | 25/15 |
| Symptom duration, years | 23.6 (13.7); [3–52] |
| Time since diagnosis, years | 9.0 (26.5) *; [0–43] |
| BMI, kg/m2 | 28.4 (7.5) *; [20.0–37.7] |
| Employed, n (%) | 23 (57.5) |
| BASMILin † | 3.8 (1.8); [1.2–7.9] |
| Lateral lumbar flexion | 4.9 (2.5); [0–9.0], (2.3cm–21.5cm) ‡ |
| Tragus to wall distance | 2.4 (2.0); [0.5–7.6], (9.5cm–30.8cm) ‡ |
| Modified Schober’s test | 5.4 (2.5); [0–9.7], (0.6cm–13.3cm) ‡ |
| Intermalleolar distance | 3.1 (1.9); [0–7.0], (55.0cm–138.5cm) ‡ |
| Cervical rotation | 3.3 (2.2); [0.3–9.5], (9.0°–87.0°) ‡ |
| Chest expansion, cm | 2.5 (2.2); [0.6–13.2] |
| Pharmacology, n (%) | |
| Anti-TNFα | 26 (65) |
| NSAIDs | 4 (10) |
| Analgesia | 4 (10) |
| None | 6 (15) |
| HLA-B27 status, n (%) | |
| Positive | 17 (42.5) |
| Negative | 7 (17.5) |
| Unknown | 16 (40) |
| BAS-G, (scale 0–10) | 3.4 (2.1) [0–7] |
| BASDAI §, (scale 0–10) | 3.4 (2.0) [0–7.9] |
| BASFI, (scale 0–10) | 3.4 (2.4) [0–8.4] |
| Method | Movement | Day 1—Supervised * | Day 1—Unsupervised † | Ambulatory ‡ | Day 2—Supervised * | Day 2—Unsupervised † |
|---|---|---|---|---|---|---|
| Trunk IMU | Flexion-Extension | 125.7 (27.1) | 121.0 (27.2) | 120.1 (27.4) | 123.7 (25.6) | 121.4 (26.4) |
| Lateral flexion L + R | 46.8 (19.7) | 45.4 (17.8) | 43.9 (18.2) | 47.1 (19.8) | 45.5 (19.0) | |
| Rotation L + R | 42.1 (22.2) | - | - | 42.2 (22.4) | - | |
| Lumbar region IMU | Flexion-Extension | 60.9 (27.0) | 58.6 (26.2) | 57.8 (25.4) | 58.2 (26.4) | 56.6 (24.9) |
| Lateral flexion L + R | 35.4 (19.1) | 34.3 (19.2) | 33.6 (19.1) | 35.4 (19.2) | 34.4 (19.0) | |
| Rotation L + R | 27.1 (16.1) | - | - | 26.8 (15.9) | - |
| Method | Movement | Day 1—Supervised * | Day 1—Unsupervised † | Ambulatory ‡ | Day 2—Supervised * | Day 2—Unsupervised † |
|---|---|---|---|---|---|---|
| Trunk IMU | Flexion-Extension | 2.2 (2.0) | 2.6 (2.0) | 2.7 (2.0) | 2.3 (2.0) | 2.5 (2.1) |
| Lateral flexion L + R | 4.0 (2.7) | 4.2 (2.4) | 4.4 (2.5) | 4.0 (2.7) | 4.2 (2.6) | |
| Rotation L + R | 3.4 (3.2) | - | - | 3.4 (3.2) | - | |
| Trunk-ASMI (Amb) | 3.2 (2.3) | 3.4 (2.1) | 3.5 (2.1) | 3.2 (2.3) | 3.3 (2.2) | |
| Lumbar region IMU | Flexion-Extension | 2.5 (2.9) | 2.6 (3.0) | 2.7 (2.9) | 2.7 (3.1) | 2.8 (3.0) |
| Lateral flexion L + R | 4.1 (3.3) | 4.3 (3.3) | 4.4 (3.3) | 4.1 (3.3) | 4.3 (3.2) | |
| Rotation L + R | 3.1 (3.3) | - | - | 3.0 (3.2) | - | |
| Lumbar-ASMI (Amb) | 3.2 (2.8) | 3.5 (3.0) | 3.5 (3.0) | 3.2 (2.9) | 3.5 (3.0) | |
| BASMILin | Total score | 3.8 (1.8) | - | - | 3.8 (1.8) | - |
| Supervised Day 1 v Supervised Day 2 * | Unsupervised Day 1 v Unsupervised Day 2 † | Supervised Day 1 v Unsupervised Day 1 † | Supervised Day 2 v Unsupervised Day 2 † | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICC [95% CI] | SEM | 95% LOA | ICC [95% CI] | SEM | 95% LOA | ICC [95% CI] | SEM | 95% LOA | ICC [95% CI] | SEM | 95% LOA | |||||||||
| Bias | Lwr | Upr | Bias | Lwr | Upr | Bias | Lwr | Upr | Bias | Lwr | Upr | |||||||||
| TrunkIMU | ||||||||||||||||||||
| Flexion + Extension | 0.93 [0.88–0.96 | 6.98 | 2.0 | −16.7 | 20.6 | 0.93 [0.88–0.96] | 7.20 | −0.3 | −19.5 | 18.8 | 0.96 [0.84–0.98] | 5.45 | 4.7 | −7.6 | 17.1 | 0.94 [0.90–0.97] | 6.00 | 2.4 | −14.1 | 19.0 |
| Lateral flexion L + R | 0.96 [0.93–0.98] | 3.95 | −0.3 | −11.4 | 10.8 | 0.95 [0.91–0.97] | 3.99 | −0.0 | −11.5 | 11.4 | 0.94 [0.89–0.97] | 4.86 | 2.5 | −18.6 | 23.7 | 0.97 [0.94–0.98] | 3.45 | 2.8 | −13.4 | 19.0 |
| Trunk-ASMI (Amb) | 0.94 [0.89–0.97] | 0.56 | −0.0 | −1.6 | 1.5 | 0.96 [0.94–0.98] | 0.42 | 0.0 | −1.15 | 1.22 | 0.91 [0.84–0.95] | 0.68 | −0.2 | −2.0 | 1.5 | 0.93 [0.86–0.96] | 0.62 | −0.1 | −1.9 | 1.6 |
| Lumbar region IMU | ||||||||||||||||||||
| Flexion + Extension | 0.89 [0.80–0.94] | 9.02 | 2.7 | −21.8 | 27.2 | 0.89 [0.80–0.94] | 8.70 | 2.0 | −21.4 | 25.4 | 0.96 [0.93–0.98] | 5.12 | 3.8 | −28.4 | 35.9 | 0.98 [0.97–0.99] | 3.57 | 0.9 | −8.7 | 10.5 |
| Lateral flexion L + R | 0.98 [0.96–0.99] | 2.84 | −0.1 | −8.1 | 7.9 | 0.97 [0.95–0.99] | 3.32 | −0.1 | −8.6 | 8.5 | 0.98 [0.97–0.99] | 2.45 | 0.7 | −6.1 | 7.5 | 0.99 [0.98–0.99] | 2.12 | 0.9 | −4.7 | 6.6 |
| Lumbar-ASMI (Amb) | 0.95 [0.90–0.97] | 0.63 | −0.0 | −1.9 | 1.8 | 0.96 [0.93–0.98] | 0.60 | −0.1 | −1.64 | 1.50 | 0.96 [0.92–0.98] | 0.57 | −0.3 | −1.9 | 1.4 | 0.96 [0.92–0.98] | 0.57 | −0.3 | −1.9 | 1.4 |
| Supervised Day 1 v Ambulatory | Unsupervised Day 1 v Ambulatory | Supervised Day 2 v Ambulatory | Unsupervised Day 2 v Ambulatory | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICC [95% CI] | SEM | 95% LOA | ICC [95% CI] | SEM | 95% LOA | ICC [95% CI] | SEM | 95% LOA | ICC [95% CI] | SEM | 95% LOA | |||||||||
| Bias | Lwr | Upr | Bias | Lwr | Upr | Bias | Lwr | Upr | Bias | Lwr | Upr | |||||||||
| Trunk IMU | ||||||||||||||||||||
| Flexion + Extension | 0.94 [0.58–0.98] | 6.56 | 6.9 | −6.1 | 19.9 | 0.97 [0.94–0.98] | 4.67 | 2.3 | −10.0 | 14.6 | 0.89 [0.78–0.94] | 8.54 | 4.7 | −18.6 | 28.0 | 0.92 [0.85–96] | 7.52 | 2.4 | −18.5 | 23.3 |
| Lateral flexion L + R | 0.94 [0.84–0.97] | 4.95 | 7.3 | −19.4 | 34.1 | 0.96 [0.93–0.98] | 3.31 | 4.8 | −18.1 | 27.7 | 0.93 [0.81–0.97] | 5.39 | 7.6 | −18.8 | 34.0 | 0.97 [0.93–0.98] | 3.45 | 4.8 | −18.8 | 28.4 |
| Trunk-ASMI (Amb) | 0.91 [0.80–0.96] | 0.68 | −0.4 | −2.1 | 1.3 | 0.97 [0.94–0.99] | 0.36 | −0.2 | −1.1 | 0.6 | 0.89 [0.78–0.94] | 0.77 | −0.3 | −2.4 | 1.7 | 0.97 [0.93–0.98] | 0.39 | −0.2 | −1.2 | 0.7 |
| Lumbar region IMU | ||||||||||||||||||||
| Flexion + Extension | 0.93 [0.86–0.96] | 7.18 | 3.6 | −14.7 | 21.8 | 0.92 [0.85–0.96] | 7.52 | 2.1 | −18.2 | 22.5 | 0.93 [0.87–0.96] | 6.82 | 0.5 | −18.3 | 19.2 | 0.93 [0.88–0.97] | 6.38 | −0.1 | −18.2 | 17.9 |
| Lateral flexion L + R | 0.98 [0.94–0.99] | 2.73 | 2.0 | −4.6 | 8.7 | 0.98 [0.97–0.99] | 2.33 | 1.2 | −4.9 | 7.4 | 0.97 [0.93–0.98] | 3.43 | 2.1 | −6.8 | 10.9 | 0.98 [0.96–0.99] | 2.61 | 1.1 | −6.1 | 8.2 |
| Lumbar-ASMI (Amb) | 0.94 [0.88–0.97] | 0.69 | −0.4 | −2.2 | 1.4 | 0.97 [0.93–0.98] | 0.52 | −0.2 | −1.7 | 1.3 | 0.95 [0.90–0.97] | 0.64 | −0.3 | −2.0 | 1.5 | 0.98 [0.97–0.99] | 0.43 | −0.1 | −1.1 | 1.0 |
| Method | Test | BASMILin Day 1 | BASMILin Day 2 |
|---|---|---|---|
| Trunk-ASMI (Amb) | Supervised Day 1 | 0.85 | 0.87 |
| Supervised Day 2 | 0.85 | 0.88 | |
| Unsupervised Day 1 | 0.85 | 0.88 | |
| Unsupervised Day 2 | 0.86 | 0.91 | |
| Ambulatory | 0.88 | 0.91 | |
| Lumbar-ASMI (Amb) | Supervised Day 1 | 0.86 | 0.88 |
| Supervised Day 2 | 0.86 | 0.86 | |
| Unsupervised Day 1 | 0.86 | 0.88 | |
| Unsupervised Day 2 | 0.86 | 0.90 | |
| Ambulatory | 0.87 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Grady, M.; O’Dwyer, T.; Connolly, J.; Condell, J.; Esquivel, K.M.; O’Shea, F.D.; Gardiner, P.; Wilson, F. Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Reliability Study in Axial Spondyloarthritis. Diagnostics 2021, 11, 490. https://doi.org/10.3390/diagnostics11030490
O’Grady M, O’Dwyer T, Connolly J, Condell J, Esquivel KM, O’Shea FD, Gardiner P, Wilson F. Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Reliability Study in Axial Spondyloarthritis. Diagnostics. 2021; 11(3):490. https://doi.org/10.3390/diagnostics11030490
Chicago/Turabian StyleO’Grady, Megan, Tom O’Dwyer, James Connolly, Joan Condell, Karla Muñoz Esquivel, Finbar D. O’Shea, Philip Gardiner, and Fiona Wilson. 2021. "Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Reliability Study in Axial Spondyloarthritis" Diagnostics 11, no. 3: 490. https://doi.org/10.3390/diagnostics11030490
APA StyleO’Grady, M., O’Dwyer, T., Connolly, J., Condell, J., Esquivel, K. M., O’Shea, F. D., Gardiner, P., & Wilson, F. (2021). Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Reliability Study in Axial Spondyloarthritis. Diagnostics, 11(3), 490. https://doi.org/10.3390/diagnostics11030490








