SPECT/CT Correlation in the Diagnosis of Unilateral Condilar Hyperplasia
Abstract
1. Introduction
2. Materials and Methods/Patients
Statistical Analysis
3. Results
Morphologic Data and Active Hyperplasia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- López, B.D.F.; Corral, S.C.M. Comparison of planar bone scintigraphy and single photon emission computed tomography for diagnosis of active condylar hyperplasia. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2016, 44, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, S.; Park, J.; Pruzansky, D.; Takagi, M.; Tai, K. A current review of asymmetry. J. Clin. Orthod. 2018, 52, 325–341. [Google Scholar] [PubMed]
- Nolte, J.; Schreurs, R.; Karssemakers, L.; Tuinzing, D.; Becking, A. Demographic features in Unilateral Condylar Hyperplasia: An overview of 309 asymmetric cases and presentation of an algorithm. J. Cranio Maxillofac. Surg. 2018, 46, 1484–1492. [Google Scholar] [CrossRef]
- Almeida, L.E.; Zacharias, J.; Pierce, S. Condylar hyperplasia: An updated review of the literature. Korean J. Orthod. 2015, 45, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Nelke, K.H.; Pawlak, W.; Morawska-Kochman, M.; Łuczak, K. Ten years of observations and demographics of hemimandibular hyperplasia and elongation. J. Cranio Maxillofac. Surg. 2018, 46, 979–986. [Google Scholar] [CrossRef]
- López Buitrago, D.F.; Ruiz Botero, J.; Corral, C.M.; Carmona, A.R.; Sabogal, A. Comparison of (99m)Tc-MDP SPECT qualitative vs. quantitative results in patients with suspected condylar hyperplasia. Rev. Española Med. Nucl. Imagen Mol. 2017, 36, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Saridin, C.P.; Raijmakers, P.G.H.M.; Tuinzing, D.B.; Becking, A.G. Bone scintigraphy as a diagnostic method in unilateral hyperactivity of the mandibular condyles: A review and meta-analysis of the literature. Int. J. Oral Maxillofac. Surg. 2011, 40, 11–17. [Google Scholar] [CrossRef]
- Shintaku, W.H.; Venturin, J.S.; Langlais, R.P.; Clark, G.T. Imaging Modalities to Access Bony Tumors and Hyperplasic Reactions of the Temporomandibular Joint. J. Oral Maxillofac. Surg. 2010, 68, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Shen, Y.; Wang, C.-Y. Clinical Value of99Tcm-MDP SPECT Bone Scintigraphy in the Diagnosis of Unilateral Condylar Hyperplasia. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Fahey, F.H.; Abramson, Z.R.; Padwa, B.L.; Zimmerman, R.E.; Zurakowski, D.; Nissenbaum, M.; Kaban, L.B.; Treves, S.T. Use of 99mTc-MDP SPECT for assessment of mandibular growth: Development of normal values. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1002–1010. [Google Scholar] [CrossRef]
- Jacene, H.A.; Goetze, S.; Patel, H.; Wahl, R.L.; Ziessman, H.A. Advantages of Hybrid SPECT/CT vs. SPECT Alone. Open Med. Imaging J. 2008, 2, 67–79. [Google Scholar] [CrossRef]
- Hamed, M.A.G.; AlAzzazy, M.Z.; Basha, M.A.A. The validity of SPECT/CT in diagnosis of condylar hyperplasia. Egypt J. Radiol. Nucl. Med. 2017, 48, 451–459. [Google Scholar] [CrossRef]
- Raijmakers, P.G.; Karssemakers, L.H.; Tuinzing, D.B. Female Predominance and Effect of Gender on Unilateral Condylar Hyperplasia: A Review and Meta-Analysis. J. Oral. Maxillofac. Surg. 2012, 70, e72–e76. [Google Scholar] [CrossRef]
- Theerakulpisut, D.; Somboonporn, C.; Wongsurawat, N. Single Photon Emission Computed Tomography without and with Hybrid Computed Tomography in Mandibular Condylar Hyperplasia. J. Med. Assoc. Thail. 2016, 99, S65–S73. [Google Scholar]
- Agarwal, K.K.; Mukherjee, A.; St, A.; Tripathi, M.; Bal, C. Incremental value of single-photon emission computed tomography/computed tomography in the diagnosis of active condylar hyperplasia. Nucl. Med. Commun. 2017, 38, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shi, J. Is Single-Photon Emission Computed Tomography/Computed Tomography Superior to Single-Photon Emission Computed Tomography in Assessing Unilateral Condylar Hyperplasia? J. Oral. Maxillofac. Surg. 2019, 77, 1279.e1–1279.e7. [Google Scholar] [CrossRef]
- López, D.F.; Botero, J.R.; Muñoz, J.M.; Cárdenas-Perilla, R.; Moreno, M. Are There Mandibular Morphological Differences in the Various Facial Asymmetry Etiologies? A Tomographic Three-Dimensional Reconstruction Study. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 2019, 77, 2324–2338. [Google Scholar] [CrossRef]
- López Buitrago, D.F.; Muñoz Acosta, J.M.; Cárdenas-Perilla, R.A. Comparison of four methods for quantitative assessment of (99m)Tc-MDP SPECT in patients with suspected condylar hyperplasia. Rev. Española Med. Nucl. Imagen Mol. 2019, 38, 72–79. [Google Scholar] [CrossRef]
- Konidena, A.; Shekhar, S.; Dixit, A.; Patil, D.J.; Gupta, R. Fusion imaging: A bipartite approach. Oral. Radiol. 2017, 34, 1–9. [Google Scholar] [CrossRef]
- Kuwert, T.; Schillaci, O. SPECT/CT: Yesterday, today, tomorrow. Clin. Transl. Imaging 2014, 2, 443–444. [Google Scholar] [CrossRef][Green Version]
- Mariani, G.; Bruselli, L.; Kuwert, T.; Kim, E.E.; Flotats, A.; Israel, O.; Dondi, M.; Watanabe, N. A review on the clinical uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1959–1985. [Google Scholar] [CrossRef]
- Wassef, H.R.; Colletti, P.M. Nuclear Medicine Imaging in the Dentomaxillofacial Region. Dent. Clin. N. Am. 2018, 62, 491–509. [Google Scholar] [CrossRef]
- Elbaz, J.; Wiss, A.; Raoul, G.; Leroy, X.; Hossein-Foucher, C.; Ferri, J. Condylar Hyperplasia. J. Craniofac. Surg. 2014, 25, 1085–1090. [Google Scholar] [CrossRef]
- Suh, M.S.; Lee, W.W.; Kim, Y.-K.; Yun, P.-Y.; Kim, S.E. Maximum Standardized Uptake Value of99mTc Hydroxymethylene Diphosphonate SPECT/CT for the Evaluation of Temporomandibular Joint Disorder. Radiology 2016, 280, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Wessels, L.; Hussain, G.; Merten, S. Discriminative Thresholds in Facial Asymmetry: A Review of the Literature. Aesthetic Surg. J. 2017, 37, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Fokoue, F.; El Mselmi, S.; Abaouz, N.; Alaoui, N.I. Added Value of SPECT-CT Imaging in the Diagnosis of Unilateral Active Mandibular Hypercondylia in Adult: A Case Report and Review. Adv. Mol. Imaging 2020, 10, 1–5. [Google Scholar] [CrossRef][Green Version]
- Verhelst, P.-J.; Shaheen, E.; Vasconcelos, K.D.F.; Van Der Cruyssen, F.; Shujaat, S.; Coudyzer, W.; Salmon, B.; Swennen, G.; Politis, C.; Jacobs, R. Validation of a 3D CBCT-based protocol for the follow-up of mandibular condyle remodeling. Dentomaxillofac. Radiol. 2020, 49, 20190364. [Google Scholar] [CrossRef]
- Nitzan, D.W.; Katsnelson, A.; Bermanis, I.; Brin, I.; Casap, N. The Clinical Characteristics of Condylar Hyperplasia: Experience with 61 Patients. J. Oral. Maxillofac. Surg. 2008, 66, 312–318. [Google Scholar] [CrossRef]
- Aarts, B.; Convens, J.; Bronkhorst, E.; Kuijpers-Jagtman, A.; Fudalej, P. Cessation of facial growth in subjects with short, average, and long facial types—Implications for the timing of implant placement. J. Cranio Maxillofac. Surg. 2015, 43, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- López Buitrago, D.F. Diferencias en la morfología ósea entre el lado desplazado y contralateral en pacientes con asimetría facial. Rev. Ces. Odontol. 2020, 2. in press. [Google Scholar]
- Farronato, M.; Cavagnetto, D.; Abate, A.; Cressoni, P.; Fama, A.; Maspero, C. Assessment of condylar volume and ramus height in JIA patients with unilateral and bilateral TMJ involvement: Retrospective case-control study. Clin. Oral Investig. 2019, 24, 2635–2643. [Google Scholar] [CrossRef]
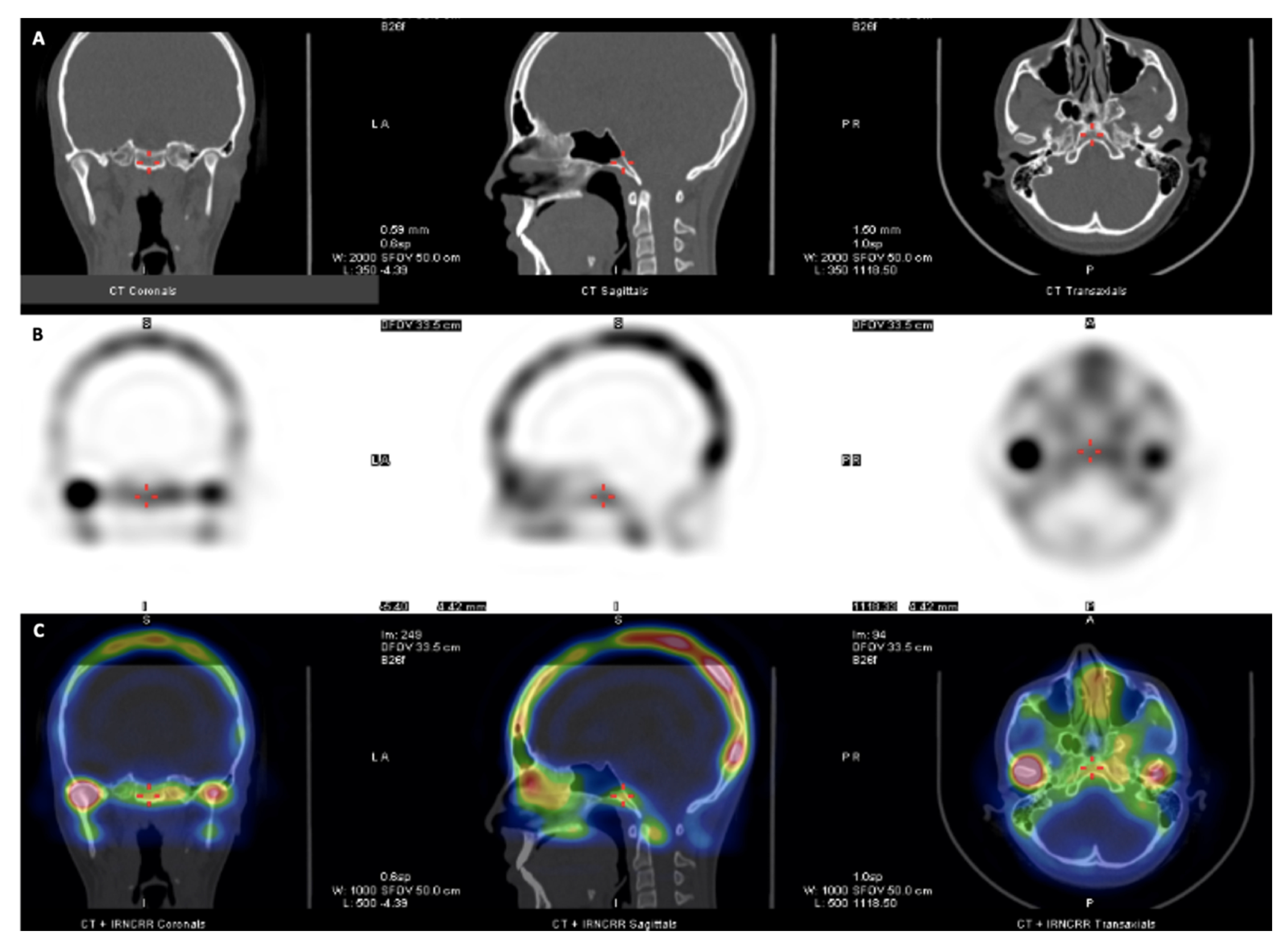



| Measurement (mm) | Description |
|---|---|
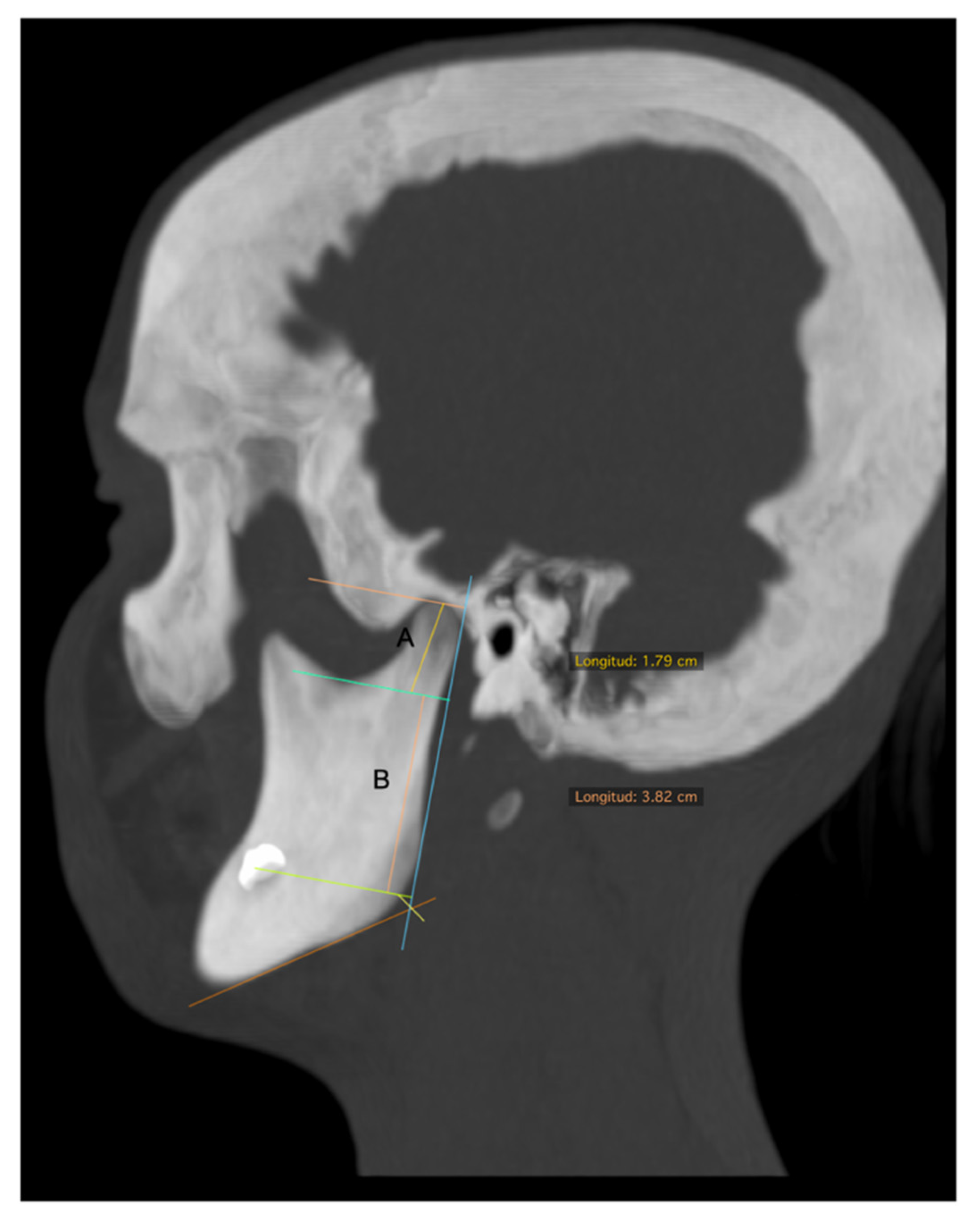
| Condylar length | In the sagittal view, a line parallel to a tangent to the posterior ridge of the mandibular ramus was traced and extended from the most superior point of the condyle to a perpendicular line, passing through the most inferior point of the mandibular notch. This length was obtained in a corrected image of the axial axis of the mandibular ramus (Figure 2A). |
| Mandibular ramus length | In the sagittal view of 3D reconstruction, a line perpendicular to the Frankfort plane, was traced and extended from the deepest point of the notch to the inferior ridge of mandibular body (Figure 2B). |
| Anteroposterior condylar length | In the axial view, a line from the most anterior point of condylar cortical bone to the most posterior limit of cortical bone was traced. This image was obtained in an orthogonal plane (Figure 3A). |
| Midlateral condylar length | In the axial view, a line from the most anterior limit of proximal cortical of the condyle to the most anterior limit of its distal cortical was traced. This image was obtained in an orthogonal plane (Figure 3B). |
| Deviation Magnitude | In the coronal view of the 3D reconstruction, repositioning the skull in a natural position of the head, the magnitude of the deviation is quantified as follows: the distance in mm from menton to the facial midline projected from the apophysis crista galli, perpendicular to the zygomatic plane was measured (Figure 4). |
| Side of mandibular deviation (laterognathism) | In the coronal view of the bone tissue 3D reconstruction, this qualitative variable was visually detected, indicating the mandibular deviation side (right/left) (Figure 4). |
| Measurement in mm | Difference * Average ± SD | Rho | p-Value |
|---|---|---|---|
| Right side total condylar length | 0.01 ± 0.19 | 0.99 (0.99–1.00) | 0.000 |
| Left side total condylar length | 0.10 ± 0.31 | 0.99 (0.99–1.00) | 0.000 |
| Right side ramus length | −0.19 ± 0.33 | 0.99 (0.99–1.00) | 0.000 |
| Left side ramus length | 0.10 ± 0.57 | 0.99 (0.99–1.00) | 0.000 |
| Anteroposterior pole of right condyle | 0.07 ± 0.19 | 0.98 (0.96–0.99) | 0.000 |
| Anteroposterior pole of left condyle | 0.19 ± 0.28 | 0.95 (0.91–0.99) | 0.000 |
| Lateral pole of right condyle | −0.19 ± 0.31 | 0.99 (0.98–1.00) | 0.000 |
| Lateral pole of left condyle | −0.05 ± 0.30 | 0.99 (−0.99–1.00) | 0.000 |
| Mandibular deviation in mm | 0.13 ± 0.51 | 0.99 (0.98–1.00) | 0.000 |
| Difference in percentage uptake | 0 ± 0 | 1 | 0.000 |
| Variable | Active n = 40 | Inactive n = 31 | Total n = 71 | p-Value |
|---|---|---|---|---|
| Age | 19 (16–26.25) | 17 (13–21.5) | 19 (15–25.5) | 0.10 |
| 22.15 (8.54) | 19.29 (7.47) | 20.90 (8.16) | 0.14 | |
| Male sex | 11 (27.5) | 13 (41.93) | 24 (33.80) | 0.22 |
| Female sex | 29 (72.5) | 18 (58.06) | 47 (66.20) | |
| Right Laterognathism | 21 (52.5) | 17 (54.84) | 38 (53.52) | 0.99 |
| Left Laterognathism | 19 (47.5) | 14 (45.16) | 33 (46.48) | |
| Difference in percentage uptake in SPECT * | 17.2 (7.52) | 3.93 (3.16) | 11.41 (8.93) | <0.001 |
| Morphologic Measurements in mm | Active | Inactive | p-Value |
|---|---|---|---|
| Right condyle total length | 19.75 (3.41) | 20.21 (3.07) | 0.55 |
| Left condyle total length | 19.95 (4.48) | 19.53 (4.88) | 0.71 |
| Right mandibular ramus length | 34.96 (5.0) | 37.29 (6.37) | 0.09 |
| Left mandibular ramus length | 34.48 (5.26) | 37.05 (5.30) | 0.05 |
| Anteroposterior pole of right condyle | 8.15 (1.15) | 7.88 (1.1) | 0.32 |
| Anteroposterior pole of left condyle | 7.72 (1.01) | 8.08 (1.2) | 0.19 |
| Midlateral pole of left condyle | 16.04 (3.41) | 15.94 (3.53) | 0.90 |
| Midlateral pole of right condyle | 15.82 (2.41) | 15.57 (3.73) | 0.73 |
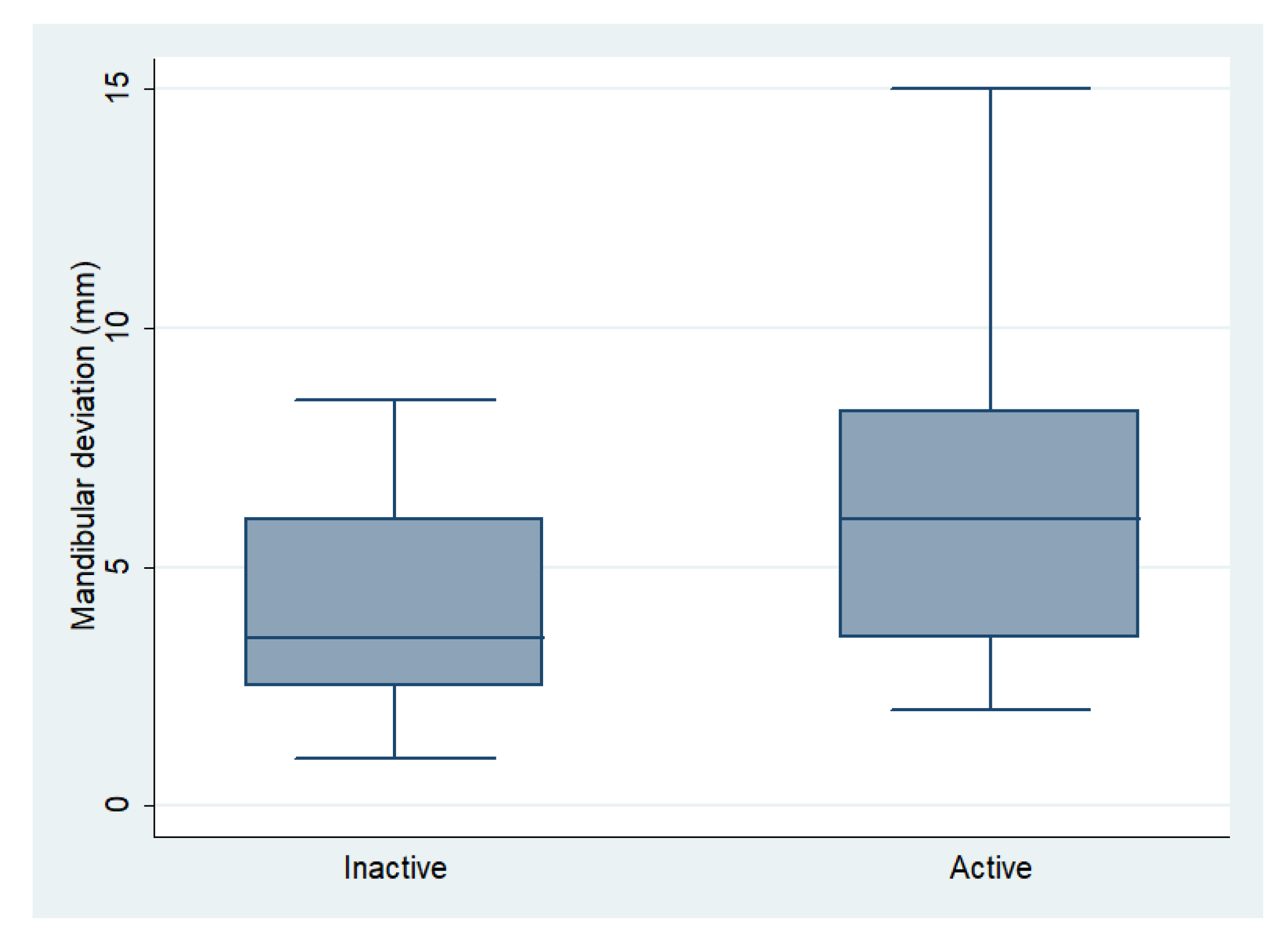
| Mandibular Deviation (mm) | 6.31 (3.46) | 4.11 (2.20) | 0.003 ** |
| Diagnostic Performance | Cut-Off Value MD = 6mm | Cut-Off Value MD = 4mm |
|---|---|---|
| TP | 22 | 30 |
| TN | 23 | 17 |
| FP | 8 | 14 |
| FN | 18 | 10 |
| Sensitivity | 55.00% | 75.00% |
| Specificity | 74.19% | 54.84% |
| Correct classification | 63.8% | 66.2% |
| PPV | 73.33% | 68.18% |
| NPV | 56.10% | 62.96% |
| LR (+) | 2.13 | 1.66 |
| LR (−) | 0.61 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, D.F.; Ríos Borrás, V.; Muñoz, J.M.; Cardenas-Perilla, R.; Almeida, L.E. SPECT/CT Correlation in the Diagnosis of Unilateral Condilar Hyperplasia. Diagnostics 2021, 11, 477. https://doi.org/10.3390/diagnostics11030477
López DF, Ríos Borrás V, Muñoz JM, Cardenas-Perilla R, Almeida LE. SPECT/CT Correlation in the Diagnosis of Unilateral Condilar Hyperplasia. Diagnostics. 2021; 11(3):477. https://doi.org/10.3390/diagnostics11030477
Chicago/Turabian StyleLópez, Diego Fernando, Valentina Ríos Borrás, Juan Manuel Muñoz, Rodrigo Cardenas-Perilla, and Luis Eduardo Almeida. 2021. "SPECT/CT Correlation in the Diagnosis of Unilateral Condilar Hyperplasia" Diagnostics 11, no. 3: 477. https://doi.org/10.3390/diagnostics11030477
APA StyleLópez, D. F., Ríos Borrás, V., Muñoz, J. M., Cardenas-Perilla, R., & Almeida, L. E. (2021). SPECT/CT Correlation in the Diagnosis of Unilateral Condilar Hyperplasia. Diagnostics, 11(3), 477. https://doi.org/10.3390/diagnostics11030477








