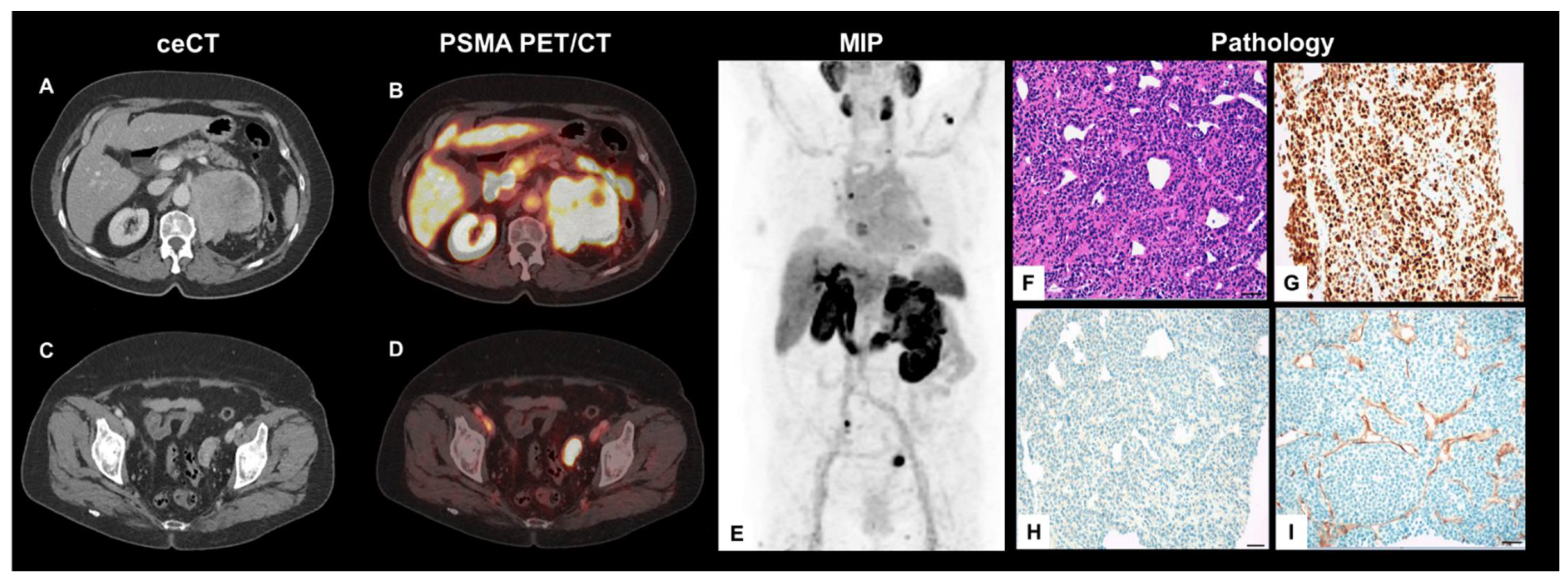
Immature Plasma Cell Myeloma Mimics Metastatic Renal Cell Carcinoma on 18F-PSMA-1007 PET/CT Due to Endothelial PSMA-Expression
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Consent for Publication
Data Availability Statement
Conflicts of Interest
References
- Meyer, A.R.; Carducci, M.A.; Denmeade, S.R.; Markowski, M.C.; Pomper, M.G.; Pierorazio, P.M.; Allaf, M.E.; Rowe, S.P.; Gorin, M.A. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 2019, 33, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Mittlmeier, L.M.; Unterrainer, M.; Todica, A.; Cyran, C.C.; Rodler, S.; Bartenstein, P.; Stief, C.G.; Ilhan, H.; Staehler, M. PSMA PET/CT for tyrosine-kinase inhibitor monitoring in metastatic renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2216–2217. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Blazak, J.; Tham, C.M.; Ng, K.L.; Shepherd, B.; Lawson, M.; Preston, J.; Vela, I.; Thomas, P.; Wood, S. Pilot study: Use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Callahan, J.; Pryor, D.; Martin, J.; Lawrentschuk, N.; Hofman, M.S. Utility of 68Ga prostate specific membrane antigen–positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J. Med. Imaging Radiat. Oncol. 2017, 61, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Mittlmeier, L.M.; Unterrainer, M.; Rodler, S.; Todica, A.; Albert, N.L.; Burgard, C.; Cyran, C.C.; Kunz, W.G.; Ricke, J.; Bartenstein, P.; et al. 18F-PSMA-1007 PET/CT for response assessment in patients with metastatic renal cell carcinoma undergoing tyrosine kinase or checkpoint inhibitor therapy: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Steiger, K.; Schwaiger, M.; Eiber, M. Image of the Month: Multifocal 68Ga Prostate-Specific Membrane Antigen Ligand Uptake in the Skeleton in a Man with Both Prostate Cancer and Multiple Myeloma. Clin. Nucl. Med. 2017, 42, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.; Joy, A.; Pillai, M.R.; Nanabala, R.; Thomas, B. 68Ga-PSMA PET/CT imaging in multiple myeloma. Clin. Nucl. Med. 2017, 42, e126–e127. [Google Scholar] [CrossRef] [PubMed]
- Veerasuri, S.; Redman, S.; Graham, R.; Meehan, C.; Little, D. Non-prostate uptake on 18F-PSMA-1007 PET/CT: A case of Myeloma. BJR Case Rep. 2020, 6, 20200102. [Google Scholar] [CrossRef]
- Pan, Q.; Luo, Y.; Ma, Y.; Li, F. The Change of 68Ga-Prostate-Specific Membrane Antigen Uptake in Myeloma After Chemotherapy in a Patient with Multiple Myeloma and Concurrent Prostate Cancer. Clin. Nucl. Med. 2020, 45, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittlmeier, L.M.; Ledderose, S.T.; Schott, M.; Brendel, M.; Beyer, L.; Theurich, S.; Mayr, D.; Walz, C.; Kunz, W.G.; Ricke, J.; et al. Immature Plasma Cell Myeloma Mimics Metastatic Renal Cell Carcinoma on 18F-PSMA-1007 PET/CT Due to Endothelial PSMA-Expression. Diagnostics 2021, 11, 423. https://doi.org/10.3390/diagnostics11030423
Mittlmeier LM, Ledderose ST, Schott M, Brendel M, Beyer L, Theurich S, Mayr D, Walz C, Kunz WG, Ricke J, et al. Immature Plasma Cell Myeloma Mimics Metastatic Renal Cell Carcinoma on 18F-PSMA-1007 PET/CT Due to Endothelial PSMA-Expression. Diagnostics. 2021; 11(3):423. https://doi.org/10.3390/diagnostics11030423
Chicago/Turabian StyleMittlmeier, Lena M., Stephan T. Ledderose, Melanie Schott, Matthias Brendel, Leonie Beyer, Sebastian Theurich, Doris Mayr, Christoph Walz, Wolfgang G. Kunz, Jens Ricke, and et al. 2021. "Immature Plasma Cell Myeloma Mimics Metastatic Renal Cell Carcinoma on 18F-PSMA-1007 PET/CT Due to Endothelial PSMA-Expression" Diagnostics 11, no. 3: 423. https://doi.org/10.3390/diagnostics11030423
APA StyleMittlmeier, L. M., Ledderose, S. T., Schott, M., Brendel, M., Beyer, L., Theurich, S., Mayr, D., Walz, C., Kunz, W. G., Ricke, J., Bartenstein, P., Ilhan, H., Staehler, M., & Unterrainer, M. (2021). Immature Plasma Cell Myeloma Mimics Metastatic Renal Cell Carcinoma on 18F-PSMA-1007 PET/CT Due to Endothelial PSMA-Expression. Diagnostics, 11(3), 423. https://doi.org/10.3390/diagnostics11030423






