Pitfalls in the Serological Diagnosis of Primary Human Cytomegalovirus Infection in Pregnancy Due to Different Kinetics of IgM Clearance and IgG Avidity Index Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Diagnosis and Timing of Primary Maternal HCMV Infection
3. Results
3.1. Characteristics of the Study Population
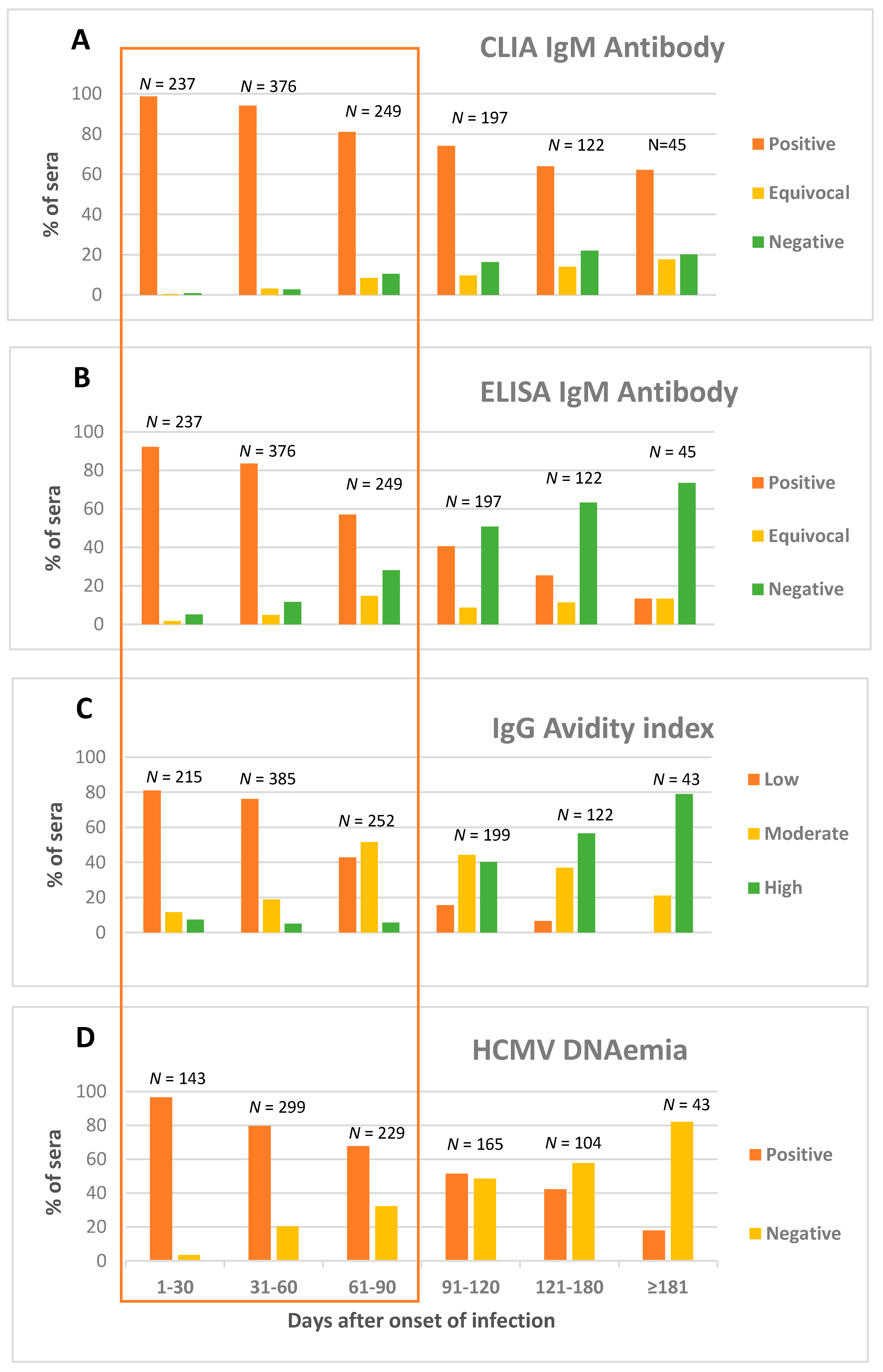
3.2. Kinetics of HCMV CLIA IgM Antibody
3.3. Kinetics of HCMV ELISA IgM Antibody
3.4. Kinetics of HCMV IgG Avidity
3.5. HCMV DNAemia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | avidity index |
| HCMV | human cytomegalovirus |
| CLIA | chemiluminescent immunoassay |
| ELISA | enzyme-linked immunosorbent assay |
| Nt | neutralizing antibodies |
References
- Cannon, M.J.; Davis, K.F. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 2005, 5, 70. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Fabbri, E.; Furione, M.; Zavattoni, M.; Lilleri, D.; Tassis, B.; Quarenghi, A.; Cena, C.; Arossa, A.; Montanari, L.; et al. Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: A 20-year experience. J. Clin. Virol. 2011, 50, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; Stagno, S.; Pass, R.F. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 2003, 289, 1008–1011. [Google Scholar] [CrossRef]
- Pass, R.F.; Fowler, K.B.; Boppana, S.B.; Britt, W.J.; Stagno, S. Congenital cytomegalovirus infection following first trimester maternal infection: Symptoms at birth and outcome. J. Clin. Virol. 2006, 35, 216–220. [Google Scholar] [CrossRef]
- Picone, O.; Vauloup-Fellous, C.; Cordier, A.G.; Guitton, S.; Senat, M.V.; Fuchs, F.; Ayoubi, J.M.; Grangeot Keros, L.; Benachi, A. A series of 238 cytomegalovirus primary infections during pregnancy: Description and outcome. Prenat. Diagn. 2013, 33, 751–758. [Google Scholar] [CrossRef]
- Zavattoni, M.; Rustico, M.; Tassis, B.; Lombardi, G.; Furione, M.; Piralla, A.; Baldanti, F. Risk of congenital disease in 46 infected fetuses according to gestational age of primary human cytomegalovirus infection in the mother. J. Med. Virol. 2016, 88, 120–126. [Google Scholar] [CrossRef]
- Sellier, Y.; Guilleminot, T.; Ville, Y.; Leruez-Ville, M. Comparison of the LIAISON(®) CMV IgG Avidity II and the VIDAS(®) CMV IgG AvidityII assays for the diagnosis of primary infection in pregnant women. J. Clin. Virol. Off. Publ. Pan. Am. Soc. Clin. Virol. 2015, 72, 46–48. [Google Scholar] [CrossRef]
- Vauloup-Fellous, C.; Lazzarotto, T.; Revello, M.G.; Grangeot-Keros, L. Clinical evaluation of the Roche Elecsys CMV IgG avidity assay. Eur. J. Clin. Microbiol. Infect Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2014, 33, 1365–1369. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Ville, Y. Fetal Infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 97–107. [Google Scholar]
- Prince, H.E.; Lapé-Nixon, M.; Brenner, A.; Pitstick, N.; Couturier, M.R. Potential impact of different cytomegalovirus (CMV) IgM assays on an algorithm requiring IgM reactivity as a criterion for measuring CMV IgG avidity. Clin. Vaccine Immunol. 2014, 21, 813–816. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Revello, M.G.; Gerna, G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 2002, 15, 680–715. [Google Scholar] [CrossRef] [PubMed]
- Lilleri, D.; Gerna, G.; Furione, M.; Zavattoni, M.; Spinillo, A. Neutralizing and ELISA IgG antibodies to human cytomegalovirus glycoprotein complexes may help date the onset of primary infection in pregnancy. J. Clin. Virol. 2016, 81, 16–24. [Google Scholar] [CrossRef]
- Revello, M.G.; Furione, M.; Rognoni, V.; Arossa, A.; Gerna, G. Cytomegalovirus DNAemia in pregnant women. J. Clin. Virol. 2014, 61, 590–592. [Google Scholar] [CrossRef]
- Revello, M.G.; Zavattoni, M.; Furione, M.; Lilleri, D.; Gorini, G.; Gerna, G. Diagnosis and management of preconceptional and periconceptional primary cytomegalovirus infection. J. Infect. Dis. 2002, 186, 55. [Google Scholar] [CrossRef]
- Razonable, R.R.; Inoue, N.; Pinninti, S.G.; Boppana, S.B.; Lazzarotto, T.; Gabrielli, L.; Simonazzi, G.; Pellett, T.E.ì.; Schmid, D.S. Clinical diagnostic testing for human cytomegalovirus infections. J. Infect Dis. 2020, 221 (Suppl. 1), S74–S85. [Google Scholar] [CrossRef]
- Furione, M.; Rognoni, V.; Sarasini, A.; Zavattoni, M.; Lilleri, D.; Gerna, G.; Revello, M.G. Slow increase in IgG avidity correlates with prevention of human cytomegalovirus transmission to the fetus. J. Med. Virol. 2013, 85, 1960–1967. [Google Scholar] [CrossRef]
- Sarasini, A.; Zavattoni, M.; Arossa, A.; Fornara, C.; Baldanti, F.; Furione, M. Kinetics of human cytomegalovirus IgG avidity maturation: A serological marker of vertical transmission. In Abstract Book; European Congenital CMV Initiative: Brussel, Belgium, 2018. [Google Scholar]
- Ebina, Y.; Minematsu, T.; Morioka, I.; Deguchi, M.; Tairaku, S.; Tanimura, K.; Sonoyama, A.; Nagamata, S.; Morizane, M.; Yamada, H. Rapid increase in the serum cytomegalovirus IgG avidity index in women with a congenitally infected fetus. J. Clin. Virol. 2015, 66, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Faure-Bardon, V.; Magny, J.F.; Parodi, M.; Couderc, S.; Garcia, P.; Maillotte, A.M.; Benard, M.; Pinquier, D.; Astruc, D.; Patural, H.; et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin. Infect Dis. 2019, 69, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Simonazzi, G.; Curti, A.; Cervi, F.; Gabrielli, L.; Contoli, M.; Capretti, M.G.; Rizzo, N.; Guerra, B.; Farina, A.; Lazzarotto, T. Perinatal outcomes of non-primary maternal cytomegalovirus infection: A 15-year experience. Fetal Diagn. Ther. 2018, 43, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.P.; Finney, J.W.; Manganello, A.M.; Best, A.M. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviours: A randomized controlled trial. Pediatr. Infect. Dis. J. 1996, 15, 240–246. [Google Scholar] [CrossRef]
- Adler, S.P.; Finney, J.W.; Manganello, A.M.; Best, A.M. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J. Pediatr. 2004, 145, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Vauloup-Fellous, C.; Picone, O.; Cordier, A.-G.; Parent-du-Châtelet, I.; Senat, M.; Frydman, R.; Grangeot-Keros, L. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J. Clin. Virol. 2009, 46S, S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Tibaldi, C.; Masuelli, G.; Frisina, V.; Sacchi, A.; Furione, M.; Arossa, A.; Spinillo, A.; Klersy, C.; Ceccarelli, M.; et al. Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine 2015, 2, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
| Pt# | Days after Onset of Infection | IgG (CLIA) * U/mL | IgM (CLIA) ° U/mL | IgM ELISA | Avidity Index | NT Titre | DNAemia § copies/mL |
|---|---|---|---|---|---|---|---|
| 1 | 12 | <12 | 28.8 | Pos | nd | <1:5 | 84 |
| 23 | 18.3 | 37.8 | Pos | Low | <1:5 | 1020 | |
| 51 | 54.9 | <18 | Neg | Low | 1:5 | 108 | |
| 81 | 66.5 | <18 | Neg | Mod | 1:10 | 30 | |
| 2 | 19 | 84.9 | 37.6 | Pos | Low | <1:5 | nd |
| 30 | 86 | 26.3 | Pos | Low | <1:5 | nd | |
| 59 | 81.1 | <18 | Neg | Low | 1:20 | 120 | |
| 88 | 79.2 | <18 | Neg | Low | 1:20 | 30 | |
| 3 | −50 | <12 | <12 | Neg | nd | <1:5 | nd |
| 21 | 50 | 65.9 | Pos | High | <1:5 | nd | |
| 56 | 78.8 | 30.2 | Pos | High | 1:10 | nd | |
| 63 | 80.9 | 27.7 | Pos | High | 1:10 | 180 | |
| 96 | 77.4 | 21.3 | Neg | High | 1:10 | 90 | |
| 4 | 16 | 41.7 | 112 | Pos | High | <1:5 | nd |
| 23 | 54.2 | 102 | Pos | High | 1:10 | 810 | |
| 58 | 73.3 | 55.5 | Pos | High | 1:20 | 30 | |
| 77 | 89.3 | 46.8 | Neg | High | 1:20 | 30 | |
| 113 | 78.3 | 39.5 | Neg | High | 1:20 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarasini, A.; Arossa, A.; Zavattoni, M.; Fornara, C.; Lilleri, D.; Spinillo, A.; Baldanti, F.; Furione, M. Pitfalls in the Serological Diagnosis of Primary Human Cytomegalovirus Infection in Pregnancy Due to Different Kinetics of IgM Clearance and IgG Avidity Index Maturation. Diagnostics 2021, 11, 396. https://doi.org/10.3390/diagnostics11030396
Sarasini A, Arossa A, Zavattoni M, Fornara C, Lilleri D, Spinillo A, Baldanti F, Furione M. Pitfalls in the Serological Diagnosis of Primary Human Cytomegalovirus Infection in Pregnancy Due to Different Kinetics of IgM Clearance and IgG Avidity Index Maturation. Diagnostics. 2021; 11(3):396. https://doi.org/10.3390/diagnostics11030396
Chicago/Turabian StyleSarasini, Antonella, Alessia Arossa, Maurizio Zavattoni, Chiara Fornara, Daniele Lilleri, Arsenio Spinillo, Fausto Baldanti, and Milena Furione. 2021. "Pitfalls in the Serological Diagnosis of Primary Human Cytomegalovirus Infection in Pregnancy Due to Different Kinetics of IgM Clearance and IgG Avidity Index Maturation" Diagnostics 11, no. 3: 396. https://doi.org/10.3390/diagnostics11030396
APA StyleSarasini, A., Arossa, A., Zavattoni, M., Fornara, C., Lilleri, D., Spinillo, A., Baldanti, F., & Furione, M. (2021). Pitfalls in the Serological Diagnosis of Primary Human Cytomegalovirus Infection in Pregnancy Due to Different Kinetics of IgM Clearance and IgG Avidity Index Maturation. Diagnostics, 11(3), 396. https://doi.org/10.3390/diagnostics11030396







