Comparison of Lung Ultrasound versus Chest X-ray for Detection of Pulmonary Infiltrates in COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design and Subjects
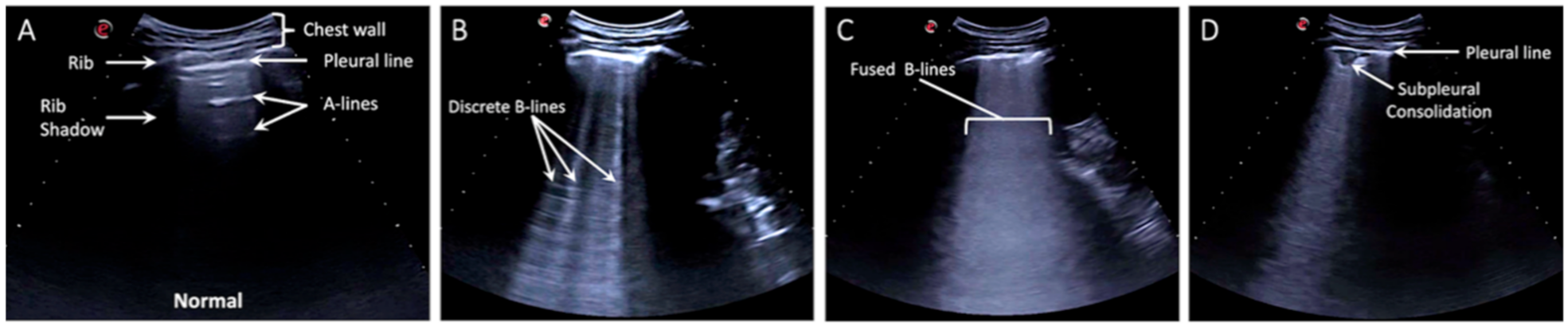
2.2. Lung Ultrasound Exam
2.3. Chest Radiographs
2.4. Statistical Analysis
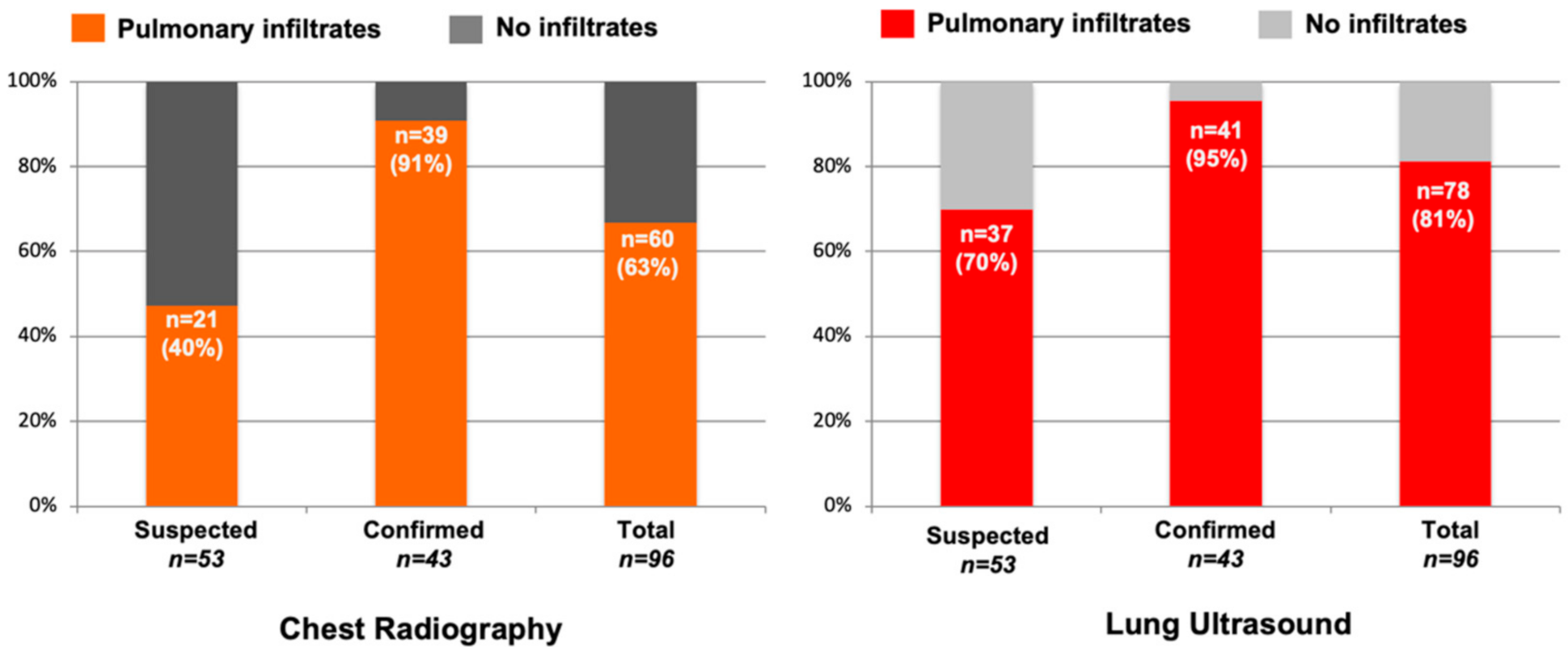
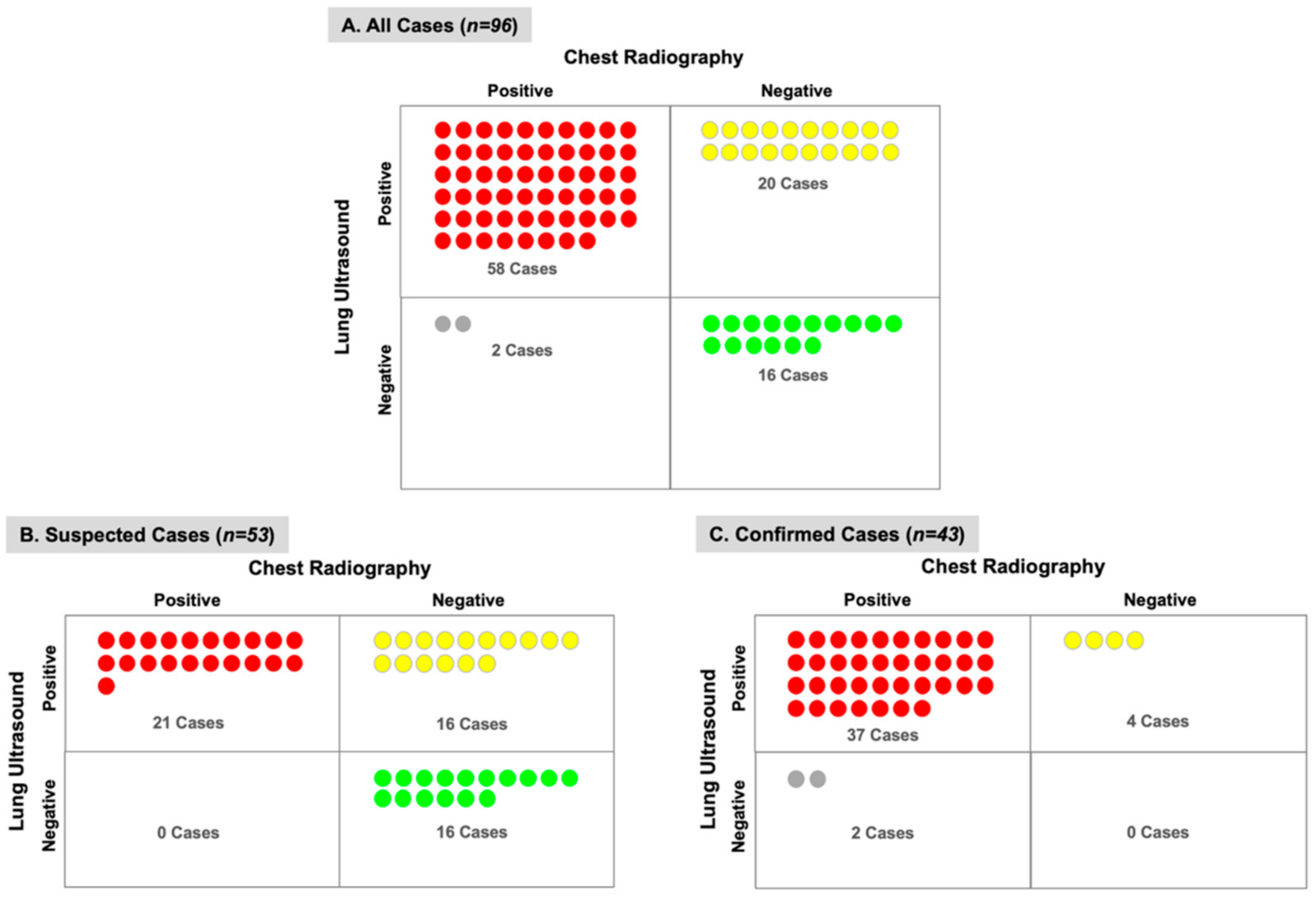
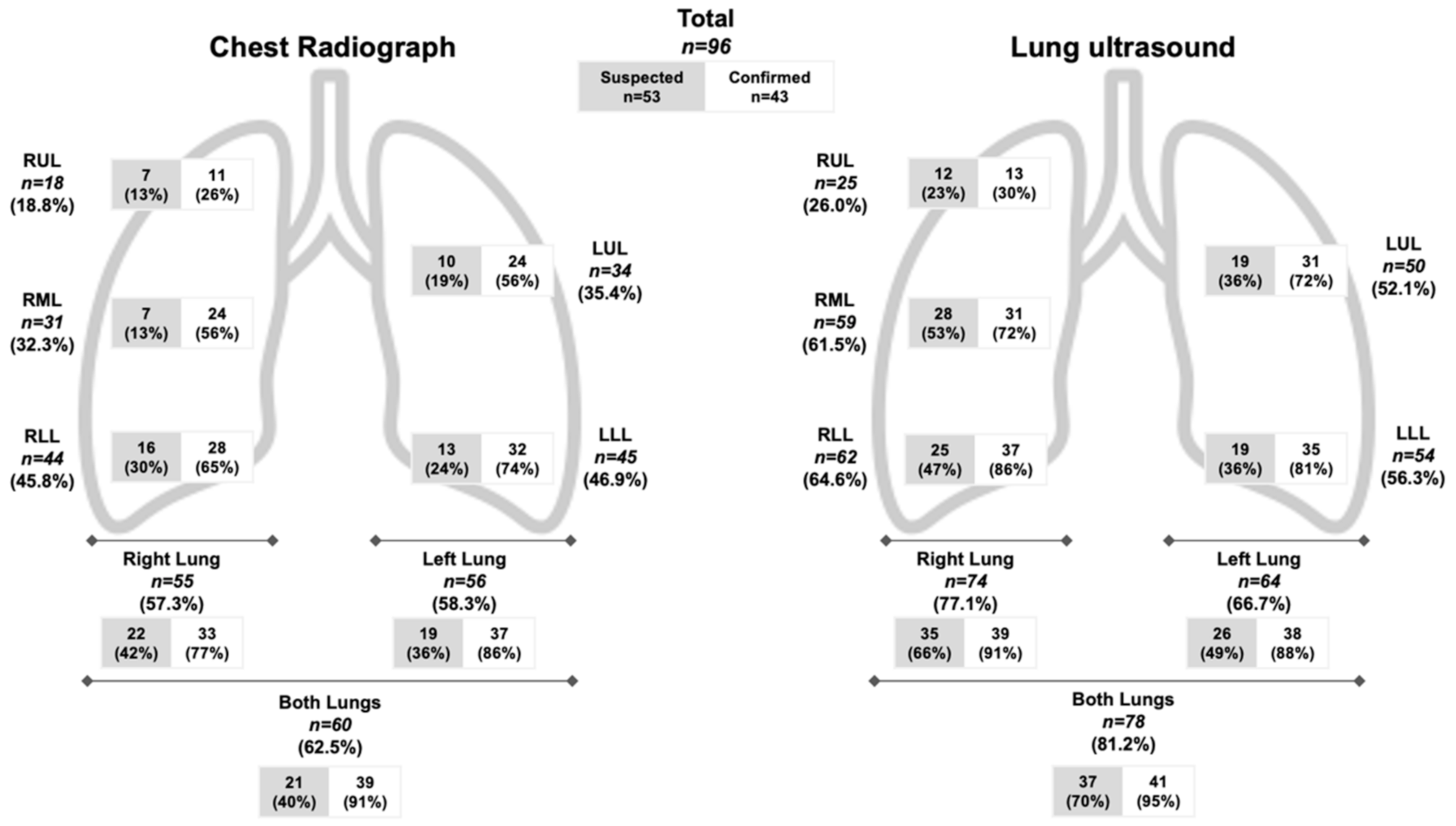
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, china. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of chest ct for covid-19: Comparison to rt-pcr. Radiology 2020, 200432. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest ct and rt-pcr testing in coronavirus disease 2019 (covid-19) in china: A report of 1014 cases. Radiology 2020, 200642. [Google Scholar] [CrossRef]
- Long, C.; Xu, H.; Shen, Q.; Zhang, X.; Fan, B.; Wang, C.; Zeng, B.; Li, Z.; Li, X.; Li, H. Diagnosis of the coronavirus disease (covid-19): Rrt-pcr or ct? Eur. J. Radiol. 2020, 126, 108961. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal changes of ct findings in 90 patients with covid-19 pneumonia: A longitudinal study. Radiology 2020, 200843. [Google Scholar] [CrossRef]
- American College or Radiology. ACR Recommendations for the Use of Chest Radiography and Computed Tomography (ct) for Suspected Covid-19 Infection. Available online: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (accessed on 10 April 2020).
- Kim, E.S.; Chin, B.S.; Kang, C.K.; Kim, N.J.; Kang, Y.M.; Choi, J.P.; Oh, D.H.; Kim, J.H.; Koh, B.; Kim, S.E.; et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: A preliminary report of the first 28 patients from the korean cohort study on covid-19. J. Korean Med. Sci. 2020, 35, e142. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.Y.F.; Lam, H.Y.S.; Fong, A.H.; Leung, S.T.; Chin, T.W.; Lo, C.S.Y.; Lui, M.M.; Lee, J.C.Y.; Chiu, K.W.; Chung, T.; et al. Frequency and distribution of chest radiographic findings in covid-19 positive patients. Radiology 2019, 201160. [Google Scholar] [CrossRef]
- Winkler, M.H.; Touw, H.R.; van de Ven, P.M.; Twisk, J.; Tuinman, P.R. Diagnostic accuracy of chest radiograph, and when concomitantly studied lung ultrasound, in critically ill patients with respiratory symptoms: A systematic review and meta-analysis. Crit. Care Med. 2018, 46, e707–e714. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, S.; Liu, Y.; Zhang, Y.; Zheng, C.; Zheng, Y.; Zhang, C.; Min, W.; Zhou, H.; Yu, M. A Preliminary Study on the Ultrasonic Manifestations of Peripulmonary Lesions of Non-Critical Novel Coronavirus Pneumonia (Covid-19). 2020. Available online: https://ssrn.com/abstract=3544750 (accessed on 15 May 2020).
- Peng, Q.Y.; Wang, X.T.; Zhang, L.N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020, 46, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Li, Q.; Du, H.; Kang, W.; Lian, J.; Yuan, L. Lung ultrasound findings in patients with covid-19 pneumonia. Crit. Care (Lond. Engl.) 2020, 24, 174. [Google Scholar] [CrossRef] [PubMed]
- Poggiali, E.; Dacrema, A.; Bastoni, D.; Tinelli, V.; Demichele, E.; Ramos, P.M.; Marcianò, T.; Silva, M.; Vercelli, A.; Magnacavallo, A. Can lung us help critical care clinicians in the early diagnosis of novel coronavirus (covid-19) pneumonia? Radiology 2020, 200847. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for international standardization of the use of lung ultrasound for covid-19 patients; a simple, quantitative, reproducible method. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Is there a role for lung ultrasound during the covid-19 pandemic? J. Ultrasound Med. 2020, 39, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Gargani, L. Sonographic signs and patterns of covid-19 pneumonia. Ultrasound J. 2020, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, K.; Minami, T. Point-of-care lung ultrasound findings in patients with novel coronavirus disease (covid-19) pneumonia. Am. J. Trop. Med. Hyg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lomoro, P.; Verde, F.; Zerboni, F.; Simonetti, I.; Borghi, C.; Fachinetti, C.; Natalizi, A.; Martegani, A. Covid-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and ct: Single-center study and comprehensive radiologic literature review. Eur. J. Radiol. Open 2020, 7, 100231. [Google Scholar] [CrossRef] [PubMed]
- Munoz, S.R.; Bangdiwala, S.I. Interpretation of kappa and b statistics measures of agreement. J. Appl. Stat. 1997, 24, 105–112. [Google Scholar] [CrossRef]
- Xiao, A.T.; Tong, Y.X.; Zhang, S. False-negative of rt-pcr and prolonged nucleic acid conversion in covid-19: Rather than recurrence. J. Med. Virol. 2020, 92, 1755–1756. [Google Scholar] [CrossRef] [PubMed]
- Mollura, D.; Lungren, M. Radiology in Global Health: Strategies, Implementation, and Applications; Springer: New York, NY, USA, 2013. [Google Scholar]
- Alzahrani, S.A.; Al-Salamah, M.A.; Al-Madani, W.H.; Elbarbary, M.A. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit. Ultrasound J. 2017, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Lamorte, A.; Villén, T. What’s new in lung ultrasound during the covid-19 pandemic. Intensive Care Med. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Suspected n = 53 n (%) | Confirmed n = 43 n (%) | Total n = 96 n (%) | p-Value |
|---|---|---|---|---|
| Gender | 0.105 | |||
| Male | 22 (41.5) | 25 (58.1) | 47 (49.0) | |
| Female | 31 (58.5) | 18 (41.9) | 49 (51.0) | |
| Age | 0.092 | |||
| Median years (IQR) | 47 (40.0–56.5) | 51 (41.0–64.0) | 48 (41.0–58.0) | |
| <30 | 5 (9.4) | 0 (0.0) | 5 (5.2) | |
| 30–39 | 8 (15.1) | 6 (14.0) | 14 (14.6) | |
| 40–49 | 18 (34.0) | 14 (32.5) | 32 (33.3) | |
| 50–59 | 13 (24.5) | 9 (20.9) | 22 (22.9) | |
| 60–69 | 7 (13.2) | 8 (18.6) | 15 (15.7) | |
| 70–79 | 2 (3.8) | 5 (11.7) | 7 (7.3) | |
| ≥80 | 0 (0.0) | 1 (2.3) | 1 (1.0) | |
| Ethnicity | 0.433 | |||
| Caucasian | 28 (52.8) | 29 (67.4) | 57 (59.4) | |
| Latin American | 17 (32.1) | 11 (25.6) | 28 (29.2) | |
| African | 5 (9.4) | 3 (7.0) | 8 (8.3) | |
| Asian | 2 (3.8) | 0 (0.0) | 2 (2.1) | |
| Other | 1 (1.9) | 0 (0.0) | 1 (1.0) | |
| Comorbidities | ||||
| Hypertension | 14 (32.6) | 12 (22.6) | 26 (27.1) | 0.277 |
| Obesity | 11 (25.6) | 9 (17.0) | 20 (20.8) | 0.302 |
| Asthma | 7 (16.3) | 5 (9.4) | 12 (12.5) | 0.313 |
| Diabetes mellitus | 4 (9.3) | 4 (7.5) | 8 (8.3) | 0.757 |
| Coronary artery disease | 1 (2.3) | 2 (3.8) | 3 (3.1) | 0.685 |
| Chronic obstructive pulmonary disease | 1 (2.3) | 2 (3.8) | 3 (3.1) | 0.685 |
| Bronchitis | 1 (2.3) | 0 (0.0) | 1 (1.0) | 0.264 |
| Human immunodeficiency virus | 1 (2.3) | 0 (0.0) | 1 (1.0) | 0.264 |
| Other | 4 (9.3) | 1 (1.9) | 5 (5.2) | 0.104 |
| Symptoms | ||||
| Fever | 43 (81.1) | 38 (88.4) | 81 (84.4) | 0.331 |
| Cough | 42 (79.2) | 37 (86.0) | 79 (82.3) | 0.385 |
| Dyspnea | 28 (52.8) | 30 (69.8) | 58 (60.4) | 0.092 |
| Myalgia | 19 (35.8) | 11 (25.6) | 30 (31.3) | 0.280 |
| Diarrhea | 11 (20.8) | 9 (20.9) | 20 (20.8) | 0.983 |
| Headache | 10 (18.9) | 2 (16.7) | 12 (12.5) | 0.036 |
| Sore throat | 7 (13.2) | 3 (7.0) | 10 (10.4) | 0.320 |
| Other | 3 (5.7) | 1 (2.3) | 4 (4.2) | 0.416 |
| Days of Symptoms | ||||
| Median days (IQR) | 6.0 (3.0–9.5) | 7.0 (5.0–10.0) | 7.0 (4.0–10.0) | 0.080 |
| <7 days | 31 (58.5) | 13 (30.2) | 44 (45.8) | 0.006 |
| ≥7 days | 22 (41.5) | 30 (57.7) | 52 (54.2) | |
| Oxygen Saturation | ||||
| Median % (IQR) | 98.0 (96–99) | 95.0 (94–97) | 97.0 (95–98) | <0.001 |
| Lung Physical Examination | 0.285 | |||
| Normal | 25 (47.2) | 25 (58.1) | 50 (52.1) | |
| Abnormal | 28 (52.8) | 18 (41.9) | 46 (47.9) | |
| Laboratory Data, median (IQR) | ||||
| Leukocytes (n = 73) (×103 /µL) | 6.1 (5.4–8.2) | 6.8 (5.3–8.4) | 6.5 (5.4–8.2) | 0.696 |
| Lymphocytes (n = 73) (×103 /µL) | 1.6 (1.2–1.9) | 1.2 (0.8–1.4) | 1.4 (1.0–1.7) | <0.001 |
| LDH (n = 71) (Nl=120–240 U/L) | 208 (160–226) | 248 (208–310) | 220 (184–275) | 0.001 |
| CRP (n = 73) (Nl < 5 mg/L) | 26 (5.0–48.0) | 51 (28.7–113.2) | 41 (12.5–91.5) | 0.001 |
| D-dimer (n = 68) (Nl < 500 ng/mL) | 455 (350–700) | 535 (405–1052) | 500 (370–757.5) | 0.170 |
| Chest X-ray | ||||
| Normal | 32 (60.3) | 4 (9.3) | 36 (37.5) | <0.001 |
| Alveolar infiltrate | 15 (28.3) | 36 (83.7) | 51 (53.1) | <0.001 |
| Interstitial infiltrate | 9 (17.0) | 12 (27.9) | 21 (21.9) | 0.198 |
| Other | 1 (1.9) | 2 (4.7) | 3 (3.1) | 0.439 |
| Lung Ultrasound Findings | ||||
| Normal | 16 (30.2) | 2 (4.7) | 18 (18.8) | 0.001 |
| Discrete B-lines | 37 (69.8) | 41 (95.3) | 78 (81.3) | 0.001 |
| Confluent B-lines | 19 (35.8) | 29 (67.4) | 48 (50.0) | 0.002 |
| Small Subpleural Consolidations (<3 cm) | 18 (34.0) | 23 (53.5) | 41 (42.7) | 0.054 |
| Large Consolidations (>3 cm) | 2 (3.8) | 0 | 2 (2.1) | 0.198 |
| Pleural effusion | 1 (1.9) | 1 (2.3) | 2 (2.1) | 0.881 |
| Other | 1 (1.9) | 1 (2.3) | 2 (2.1) | 0.881 |
| Disposition | <0.001 | |||
| Hospitalized | 3 (5.7) | 35 (81.4) | 38 (39.6) | |
| Home with Close Follow-up | 50 (94.3) | 8 (18.6) | 58 (60.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos González, M.; García de Casasola Sánchez, G.; Muñoz, F.J.T.; Proud, K.; Lourdo, D.; Sander, J.-V.; Jaimes, G.E.O.; Mader, M.; Canora Lebrato, J.; Restrepo, M.I.; et al. Comparison of Lung Ultrasound versus Chest X-ray for Detection of Pulmonary Infiltrates in COVID-19. Diagnostics 2021, 11, 373. https://doi.org/10.3390/diagnostics11020373
Mateos González M, García de Casasola Sánchez G, Muñoz FJT, Proud K, Lourdo D, Sander J-V, Jaimes GEO, Mader M, Canora Lebrato J, Restrepo MI, et al. Comparison of Lung Ultrasound versus Chest X-ray for Detection of Pulmonary Infiltrates in COVID-19. Diagnostics. 2021; 11(2):373. https://doi.org/10.3390/diagnostics11020373
Chicago/Turabian StyleMateos González, María, Gonzalo García de Casasola Sánchez, Francisco Javier Teigell Muñoz, Kevin Proud, Davide Lourdo, Julia-Verena Sander, Gabriel E. Ortiz Jaimes, Michael Mader, Jesús Canora Lebrato, Marcos I. Restrepo, and et al. 2021. "Comparison of Lung Ultrasound versus Chest X-ray for Detection of Pulmonary Infiltrates in COVID-19" Diagnostics 11, no. 2: 373. https://doi.org/10.3390/diagnostics11020373
APA StyleMateos González, M., García de Casasola Sánchez, G., Muñoz, F. J. T., Proud, K., Lourdo, D., Sander, J.-V., Jaimes, G. E. O., Mader, M., Canora Lebrato, J., Restrepo, M. I., & Soni, N. J. (2021). Comparison of Lung Ultrasound versus Chest X-ray for Detection of Pulmonary Infiltrates in COVID-19. Diagnostics, 11(2), 373. https://doi.org/10.3390/diagnostics11020373








