Association of Multiple Glycemic Parameters at Hospital Admission with Mortality and Short-Term Outcomes in Acutely Poisoned Patients
Abstract
1. Introduction
2. Materials and Methods
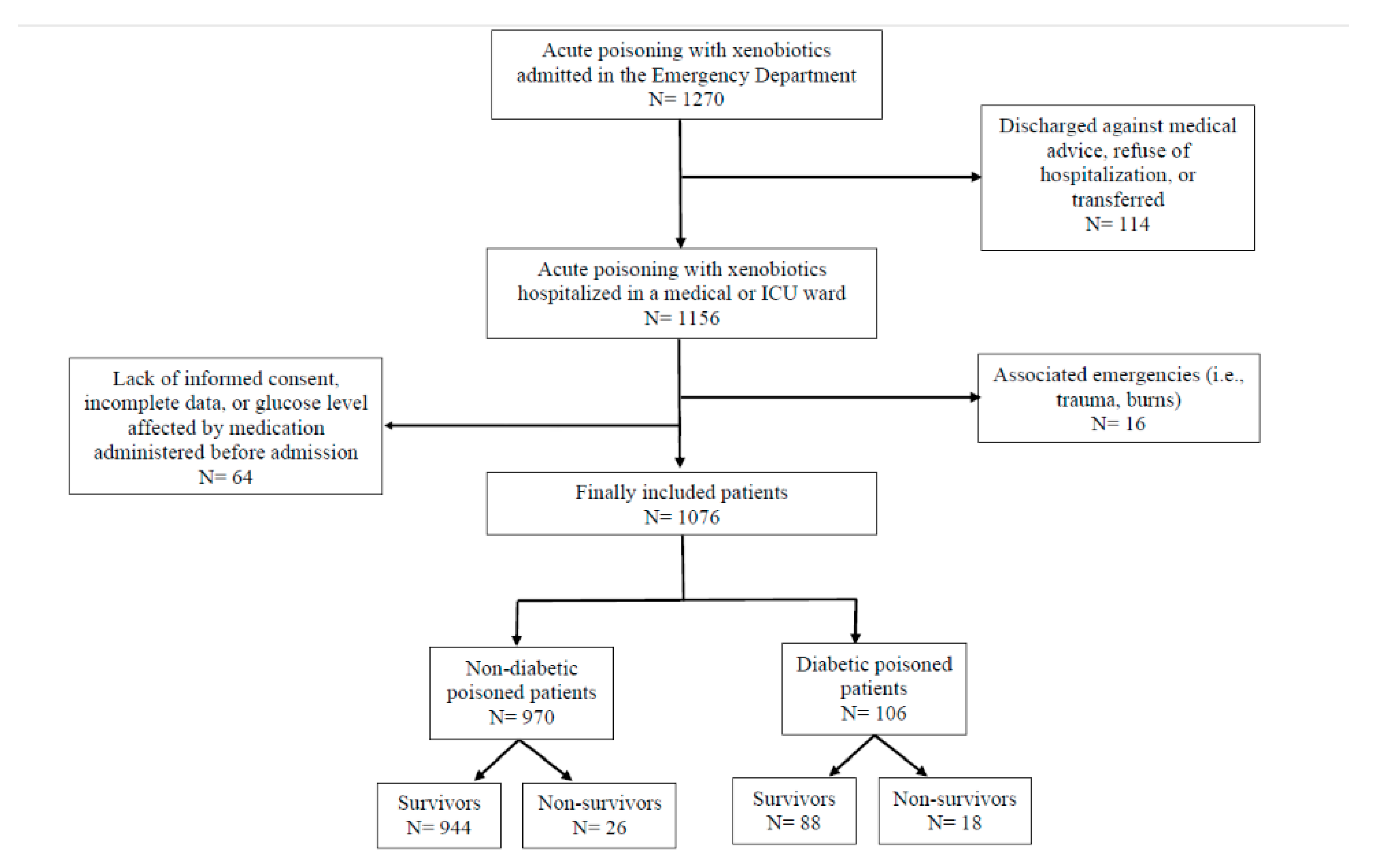
2.1. Study Population
2.2. Baseline Data Collection
2.3. Outcomes and Definitions
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
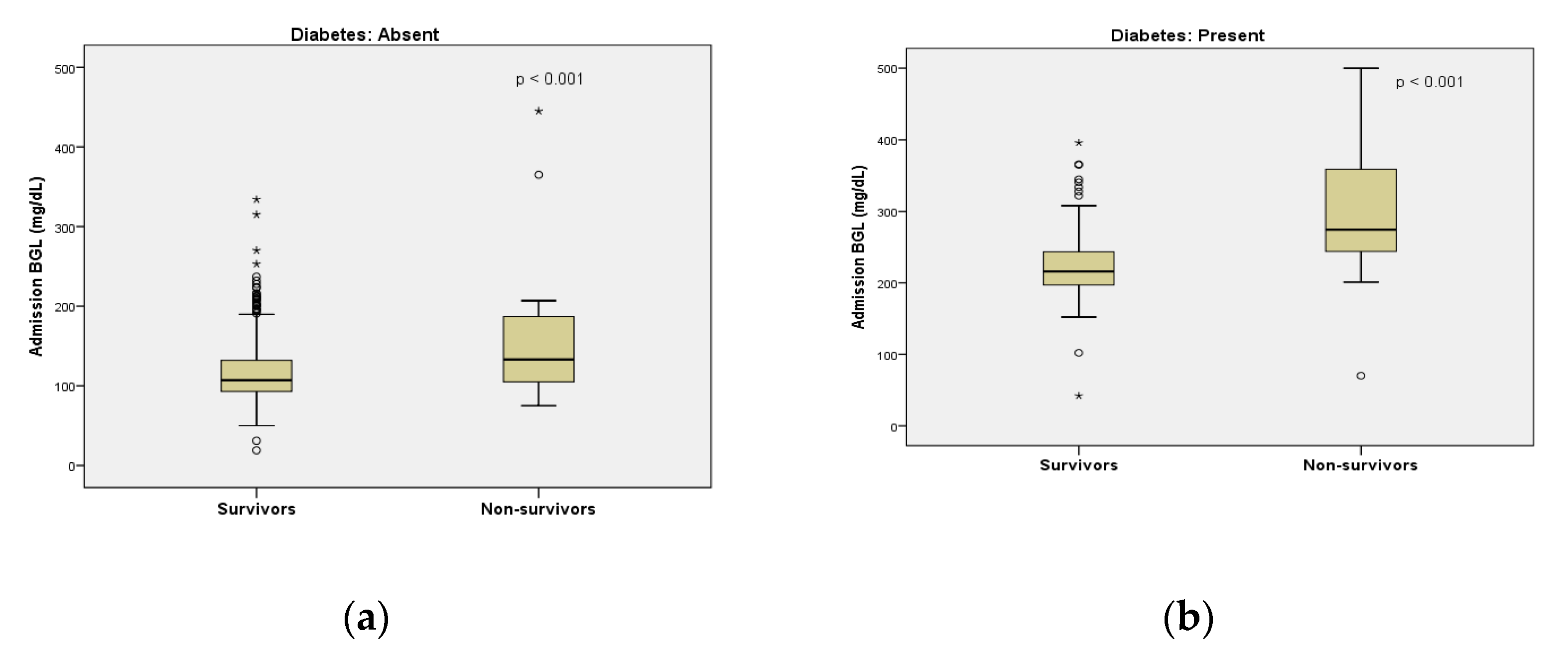
3.2. Admission Blood Glucose Level and Outcomes
3.3. Other Glycemic Parameters and Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boord, J.B.; Greevy, R.A.; Braithwaite, S.S.; Arnold, P.C.; Selig, P.M.; Brake, H.; Cuny, J.; Baldwin, D. Evaluation of hospital glycemic control at US Academic Medical Centers. J. Hosp. Med. 2009, 4, 35–44. [Google Scholar] [CrossRef]
- Zaccardi, F.; Dhalwani, N.N.; Webb, D.R.; Davies, M.J.; Khunti, K. Global burden of hypoglycaemia-related mortality in 109 countries, from 2000 to 2014: An analysis of death certificates. Diabetologia 2018, 61, 1592–1602. [Google Scholar] [CrossRef]
- Garadah, T.S.; Kassab, S.; Al-Shboul, Q.M.; Alawadi, A. The Threshold of Admission Glycemia as a Predictor of Adverse Events in Diabetic and Non-Diabetic Patients with Acute Coronary Syndrome. Clin. Med. Cardiol. 2009, 3, 29–36. [Google Scholar] [CrossRef]
- Foltran, F.; Gregori, D.; Caropreso, A.; Pagano, E.; Bruno, A. Is blood glucose on admission a predictor of mortality in adult acute pneumonia? Clin. Respir. J. 2013, 7, 276–280. [Google Scholar] [CrossRef]
- Baker, E.H.; Janaway, C.H.; Philips, B.J.; Brennan, A.L.; Baines, D.L.; Wood, D.M.; Jones, P.W. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax 2006, 61, 284–289. [Google Scholar] [CrossRef]
- Krinsley, J.S. Glycemic Variability and Mortality in Critically Ill Patients: The Impact of Diabetes. J. Diabetes Sci. Technol. 2009, 3, 1292–1301. [Google Scholar] [CrossRef]
- Akirov, A.; Diker-Cohen, T.; Masri-Iraqi, H.; Shimon, I. High glucose variability increases mortality risk in hospitalized patients. J. Clin. Endocrinol. Metab. 2017, 102, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Shohat, N.; Foltz, C.; Restrepo, C.; Goswami, K.; Tan, T.; Parvizi, J. Increased postoperative glucose variability is associated with adverse outcomes following orthopaedic surgery. Bone Joint. J. 2018, 100, 1125–1132. [Google Scholar] [CrossRef]
- Jones, A.F. Metabolic effects of poisoning. Medicine 2016, 44, 87–90. [Google Scholar] [CrossRef]
- Hermanides, J.; Vriesendorp, T.M.; Bosman, R.J.; Zandstra, D.F.; Hoekstra, J.B.; DeVries, J.H. Glucose variability is associated with intensive care unit mortality. Crit. Care. Med. 2010, 38, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, X.; Wei, J.; Mo, H.; Lou, H.; Gong, N.; Zhang, M. Effects of Glucose Variability on Short-Term Outcomes in Non-Diabetic Patients After Coronary Artery Bypass Grafting: A Retrospective Observational Study. Heart Lung Circ. 2019, 28, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Meynaar, I.A.; Eslami, S.; Abu-Hanna, A.; van der Voort, P.; de Lange, D.W.; de Keizer, N. Blood glucose amplitude variability as predictor for mortality in surgical and medical intensive care unit patients: A multicenter cohort study. J. Crit. Care. 2012, 27, 119–124. [Google Scholar] [CrossRef]
- Dungan, K.M.; Binkley, P.; Nagaraja, H.N.; Schuster, D.; Osei, K. The effect of glycaemic control and glycaemic variability on mortality in patients hospitalized with congestive heart failure. Diabetes Metab. Res. Rev. 2011, 27, 85–93. [Google Scholar] [CrossRef]
- Sanaei-Zadeh, H.; Esfeh, S.K.; Zamani, N.; Jamshidi, F.; Shadnia, S. Hyperglycemia Is a Strong Prognostic Factor of Lethality in Methanol Poisoning. J. Med. Toxicol. 2011, 7, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.M.; Chun, B.J.; Cho, Y.S. Hyperglycemia at presentation is associated with in hospital mortality in non-diabetic patient with organophosphate poisoning. Clin. Toxicol. 2016, 54, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Sabzghabaee, A.M.; Eizadi-Mood, N.; Gheshlaghi, F.; Adib, N.; Safaeian, L. Is there a relationship between admission blood glucose level following acute poisoning and clinical outcome? Arch. Med. Sci. 2011, 7, 81–86. [Google Scholar] [CrossRef]
- Penney, D.G. Hyperglycemia exacerbates brain damage in acute severe carbon monoxide poisoning. Med. Hypotheses 1988, 27, 241–244. [Google Scholar] [CrossRef]
- Viana, M.V.; Moraes, R.B.; Fabbrin, A.R.; Santos, M.F.; Gerchman, F. Avaliação e tratamento da hiperglicemia em pacientes graves [Assessment and treatment of hyperglycemia in critically ill patients]. Rev. Bras. Ter. Intensive. 2014, 26, 71–76. [Google Scholar] [CrossRef]
- Nikkanen, H.E.; Shannon, M.W. Endocrine toxicology. In Haddad and Winchester’s Clinical management of Poisoning and Drug Overdose, 4th ed.; Shannon, M.W., Borron, S.W., Burns, M.J., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2007; pp. 317–346. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; Mackenzie, R.C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Persson, H.E.; Sjoberg, G.K.; Haines, J.A.; Pronczuk de Garbino, J. Poisoning severity score. Grading of acute poisoning. J. Toxicol. Clin. Toxicol. 1998, 36, 205–213. [Google Scholar] [CrossRef]
- Casey, P.B.; Dexter, E.M.; Michell, J.; Vale, J.A. The prospective value of the IPCS/EC/EAPCCT poisoning severity score in cases of poisoning. J. Toxicol. Clin. Toxicol. 1998, 36, 215–217. [Google Scholar] [CrossRef]
- Moebus, S.; Göres, L.; Lösch, C.; Jöckel, K.H. Impact of time since last caloric intake on blood glucose levels. Eur. J. Epidemiol. 2011, 26, 719–728. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S173–S181. [Google Scholar] [CrossRef]
- Kavanagh, B.P.; McCowen, K.C. Clinical practice. Glycemic control in the ICU. N. Engl. J. Med. 2010, 363, 2540–2546. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.N.; O’Brien, M.J., Jr.; Dungan, K.; Phillips, G.; Marsh, B.C.; Lemeshow, S.; Connors, F.A., Jr.; Preiser, C.J. Glucose variability and mortality in patients with sepsis. Crit. Care. Med. 2008, 36, 2316–2321. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–967. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Lionte, C.; Sorodoc, L.; Laba, V. Toxic-induced hypoglycemia in clinical practice. Rom. J. Intern. Med. 2004, 42, 447–455. [Google Scholar] [PubMed]
- Mehrpour, O.; Alfred, S.; Shadnia, S.; Keyler, D.E.; Soltaninejad, K.; Chalaki, N.; Sedaghat, M. Hyperglycemia in acute aluminum phosphide poisoning as a potential prognostic factor. Hum. Exp. Toxicol. 2008, 27, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Abdollahi, M. A review on the mechanisms involved in hyperglycemia induced by organophosphorus pesticides. Pestic. Biochem. Physiol. 2007, 88, 115–121. [Google Scholar] [CrossRef]
- Lee, B.K.; Lee, H.Y.; Jeung, K.W.; Jung, Y.H.; Lee, G.S.; You, Y. Association of blood glucose variability with outcomes in comatose cardiac arrest survivors treated with therapeutic hypothermia. Am. J. Emerg. Med. 2013, 31, 566–572. [Google Scholar] [CrossRef]
- Wang, S.H.; Xu, C.; Tan, T.L.; Goswami, K.; Cooper, A.M.; Parvizi, J. Increased Postoperative Glucose Variability Is Associated With Adverse Outcome Following Two-Stage Exchange Arthroplasty for Periprosthetic Joint Infection. J. Arthroplast. 2020, 35, 1368–1373. [Google Scholar] [CrossRef]
- Winter, W.E.; Pittman, D.L.; Devaraj, S.; Li, D.; Harris, N.S. Evaluation of hyperglycemia. In Handbook of Diagnostic Endocrinology, 3rd ed.; Winter, W.E., Sokoll, L.J., Holmquist, B., Bertholf, R.L., Eds.; Academic Press: London, UK, 2021; pp. 237–286. [Google Scholar] [CrossRef]
- Kunnummal Madathodi, A.R.; Andrews, M.; Madhavan, I. Ethylene Glycol Poisoning; an Unusual Cause of Hyperglycemia: A Case Report. Asia. Pac. J. Med. Toxicol. 2015, 4, 55–57. [Google Scholar] [CrossRef]
- Quach, S.; Hennessy, D.A.; Faris, P.; Fong, A.; Quan, H.; Doig, C. A comparison between the APACHE II and Charlson Index Score for predicting hospital mortality in critically ill patients. BMC Health Serv. Res. 2009, 9, 129. [Google Scholar] [CrossRef]
- Yalin, S.F.; Bakir, A.; Trabulus, S.; Seyahi, N.; Altiparmak, M.R. The Charlson Comorbidity Index: Can it predict the outcome in acute kidney injury? Int. Urol. Nephrol. 2020, 52, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, P.; Schaeffer, A.F.; Dullius, D.P.; Viana, M.V.; Leitão, C.B.; Rech, T.H. Association of multiple glycemic parameters at intensive care unit admission with mortality and clinical outcomes in critically ill patients. Sci. Rep. 2019, 9, 18498. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Oliver, N.S.; Choudhary, P.; Levy, J.C.; Hindmarsh, P.; Matthews, D.R. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol. Ther. 2011, 13, 921–928. [Google Scholar] [CrossRef]
- Sim, J.; Reid, N. Statistical inference by confidence intervals: Issues of interpretation and utilization. Phys. Ther. 1999, 79, 186–195. [Google Scholar] [CrossRef]
| Parameter | Nondiabetic Poisoned Patients (n = 970) | p-Value * | Diabetic Poisoned Patients (n = 106) | p-Value |
|---|---|---|---|---|
| Age (years) | 44 [32–60] | <0.001 | 57 [42–67.25] | 0.083 |
| CCI score (S/N, %) | 0.757 | 0.896 | ||
| CCI 0 | 96.8/3.2 | - | ||
| CCI 1–2 | 97.9/2.1 | 83.1/16.9 | ||
| CCI 3–4 | 98.1/1.9 | 85.2/14.8 | ||
| CCI ≥ 5 | 97.7/2.3 | 80/20 | ||
| Poison type (S/N, %) | 0.422 | 0.021 | ||
| Combination of poisons | 26.8/0.8 | 13.2/0 | ||
| Drugs/medicines | 38.2/0.7 | 29.2/2.8 | ||
| Non-pharmaceuticals | 32.2/1.1 | 40.6/14.2 | ||
| GCS (S/N, %) | 0.002 | 0.001 | ||
| ≥ 8 | 81.6/1.5 | 58.5/4.7 | ||
| < 8 | 15.7/1.1 | 24.5/12.3 | ||
| PSS (S/N, %) | <0.001 | <0.001 | ||
| Minor | 44.3/0 | 25/0 | ||
| Moderate | 42.6/30.8 | 53.4/11.1 | ||
| Severe | 12.8/65.4 | 21.6/55.6 | ||
| Fatal | 0.2/3.8 | 0/33.3 | ||
| SBP (mmHg) | 125 [110–140] | 0.018 | 135 [104–153.5] | <0.001 |
| HR (bpm) | 84 [73–100] | 0.048 | 91 [75–114] | 0.147 |
| pH | 7.39 [7.35–7.43] | 0.837 | 7.37 [7.25–7.41] | <0.001 |
| K+ (mmol/L) | 4 [3.7–4.3] | 0.984 | 3.9 [3.4–4.43] | <0.001 |
| CRP (mg/dL) | 0.37 [0.12–1.49] | <0.001 | 0.59 [0.15–1.92] | 0.189 |
| Hb (g/dL) | 13.70 [12.5–14.9] | 0.279 | 13.4 [12.4–14.53] | 0.612 |
| BGL (mg/dL) | 109 [93–132] | <0.001 | 221.5 [200.5–266.25] | <0.001 |
| MGL (mg/dL) | 109 [94.58–136.25] | 0.386 | 112.84 [94.92–140.19] | 0.935 |
| SD (mg/dL) | 12.02 [4.51–29.16] | 0.759 | 13.20 [4.95–27.93] | 0.658 |
| CV (%) | 0.11 [0.04–0.24] | 0.781 | 0.12 [0.05–0.21] | 0.915 |
| MAGE (mg/dL) | 28 [7–94.25] | 0.899 | 40 [9–117.75] | 0.831 |
| MAG (mg/dL/h) | 13 [6–28] | 0.532 | 13.55 [5.7–27.31] | 0.696 |
| Creatinine (mg/dL) | 0.77 [0.69–0.90] | <0.001 | 0.83 [0.73–1.05] | <0.001 |
| ALAT (U/L) | 20 [14–32] | 0.133 | 27 [17–48.5] | 0.044 |
| ICU therapy (S/N, %) | <0.001 | <0.001 | ||
| No | 82.9/0.2 | 60/1.9 | ||
| Yes | 14.4/2.5 | 22.9/15.2 | ||
| ICU hospitalization (days) | 4 [3–6] | <0.001 | 5 [3–7.25] | 0.631 |
| Poison Involved | Nondiabetic Patients (n = 970) | p-Value | Diabetic Patients (n = 106) | p-Value |
|---|---|---|---|---|
| Prescription drugs | 107.67 ± 25.899 | <0.001 | 233.72 ± 52.949 | 0.066 |
| Combination of poisons | 110.54 ± 27.422 | 194.79 ± 61.481 | ||
| Pesticides | 144.05 ± 54.999 | 227.75 ± 70.404 | ||
| Caustic agents | 115.26 ± 33.349 | 244.80 ± 80.372 | ||
| Toxic alcohols and chemicals | 130.86 ± 56.109 | 280.45 ± 90.574 | ||
| Toxic gases | 127.77 ± 31.167 | 257.14 ± 57.389 | ||
| OTC | 110.65 ± 24.245 | 231.00 ± 46.669 | ||
| Plant toxins | 125.45 ± 28.346 | 203.33 ± 19.009 | ||
| Drugs of abuse | 120.40 ± 33.721 | 228.33 ± 98.083 |
| Variable | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age | 1.065 | 1.033–1.098 | <0.001 | 1.065 | 1.033–1.098 | <0.001 |
| GCS < 8 | 0.174 | 0.094–0.321 | <0.001 | 2.774 | 0.933–8.244 | 0.066 |
| CRP | 1.066 | 1.023–1.111 | 0.003 | 0.992 | 0.933–1.055 | 0.804 |
| BGL | 1.015 | 1.011–1.019 | <0.001 | 1.007 | 1.002–1.013 | 0.005 |
| ICU therapy | 0.019 | 0.007–0.054 | <0.001 | 0.021 | 0.005–0.088 | <0.001 |
| Creatinine | 1.650 | 1.230–2.212 | 0.001 | 1.176 | 0.813–1.699 | 0.389 |
| Lactate | 1.480 | 1.35–1.62 | <0.001 | 1.349 | 1.199–1.517 | <0.001 |
| General Outcome a | B | Std. Error | Wald | p-Value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Moderate | Age | 0.009 | 0.005 | 3.666 | 0.056 | 1.009 | 1.000–1.018 |
| Admission BGL | 0.005 | 0.002 | 5.451 | 0.020 | 1.005 | 1.001–1.009 | |
| Creatinine | 0.725 | 0.354 | 4.205 | 0.040 | 2.065 | 1.033–4.131 | |
| Lactate | 0.086 | 0.044 | 3.796 | 0.051 | 1.090 | 0.999–1.188 | |
| GCS > 8 | −1.689 | 0.426 | 15.695 | 0.000 | 0.185 | 0.080–0.426 | |
| No ICU therapy | −1.259 | 0.433 | 8.448 | 0.004 | 0.284 | 0.121–0.664 | |
| Poor | Age | 0.076 | 0.017 | 20.068 | 0.000 | 1.079 | 1.044–1.116 |
| Admission BGL | 0.013 | 0.004 | 14.105 | 0.000 | 1.013 | 1.006–1.020 | |
| Creatinine | 0.854 | 0.390 | 4.802 | 0.028 | 2.348 | 1.094–5.040 | |
| CV | 6.758 | 3.653 | 3.422 | 0.064 | 860.937 | 0.669–1,107,985.854 | |
| MAG | −0.048 | 0.026 | 3.310 | 0.069 | 0.954 | 0.906–1.004 | |
| Lactate | 0.385 | 0.074 | 26.759 | 0.000 | 1.469 | 1.270–1.700 | |
| No ICU therapy | −5.220 | 0.866 | 36.349 | 0.000 | 0.005 | 0.001–0.030 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lionte, C.; Bologa, C.; Agafiti, I.; Sorodoc, V.; Petris, O.R.; Jaba, E.; Sorodoc, L. Association of Multiple Glycemic Parameters at Hospital Admission with Mortality and Short-Term Outcomes in Acutely Poisoned Patients. Diagnostics 2021, 11, 361. https://doi.org/10.3390/diagnostics11020361
Lionte C, Bologa C, Agafiti I, Sorodoc V, Petris OR, Jaba E, Sorodoc L. Association of Multiple Glycemic Parameters at Hospital Admission with Mortality and Short-Term Outcomes in Acutely Poisoned Patients. Diagnostics. 2021; 11(2):361. https://doi.org/10.3390/diagnostics11020361
Chicago/Turabian StyleLionte, Catalina, Cristina Bologa, Inga Agafiti, Victorita Sorodoc, Ovidiu Rusalim Petris, Elisabeta Jaba, and Laurentiu Sorodoc. 2021. "Association of Multiple Glycemic Parameters at Hospital Admission with Mortality and Short-Term Outcomes in Acutely Poisoned Patients" Diagnostics 11, no. 2: 361. https://doi.org/10.3390/diagnostics11020361
APA StyleLionte, C., Bologa, C., Agafiti, I., Sorodoc, V., Petris, O. R., Jaba, E., & Sorodoc, L. (2021). Association of Multiple Glycemic Parameters at Hospital Admission with Mortality and Short-Term Outcomes in Acutely Poisoned Patients. Diagnostics, 11(2), 361. https://doi.org/10.3390/diagnostics11020361






