Paravertebral Muscle Mechanical Properties and Spinal Range of Motion in Patients with Acute Neck or Low Back Pain: A Case-Control Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size
2.4. Assessments and Procedures
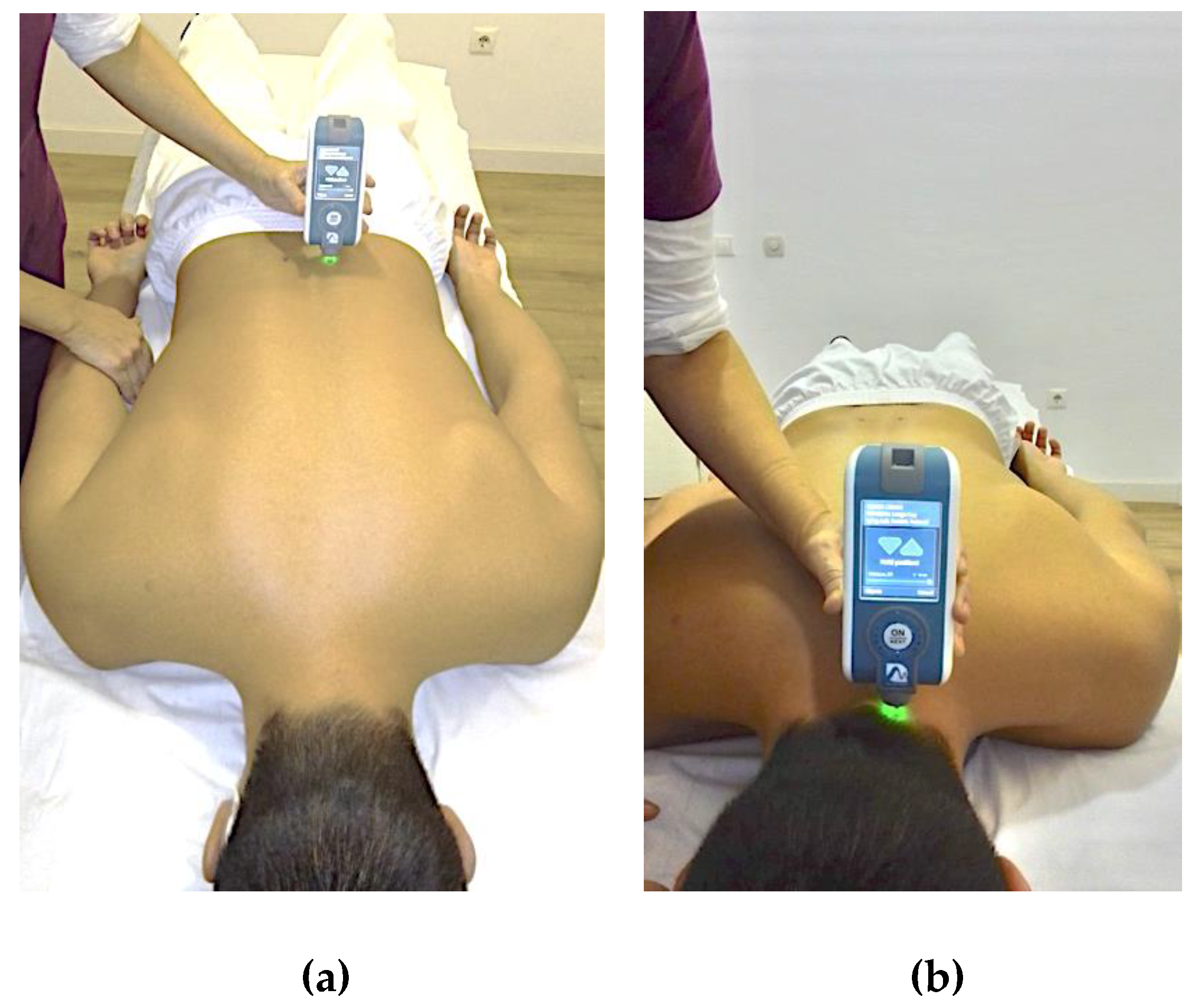
2.4.1. Assessment of Muscle Mechanical Properties (MMPs)
2.4.2. Range of Motion (ROM) Assessment
2.4.3. Self-Reported Questionnaires
2.5. Statistical Analysis
3. Results
3.1. Differences in MMPs and ROMs among Groups
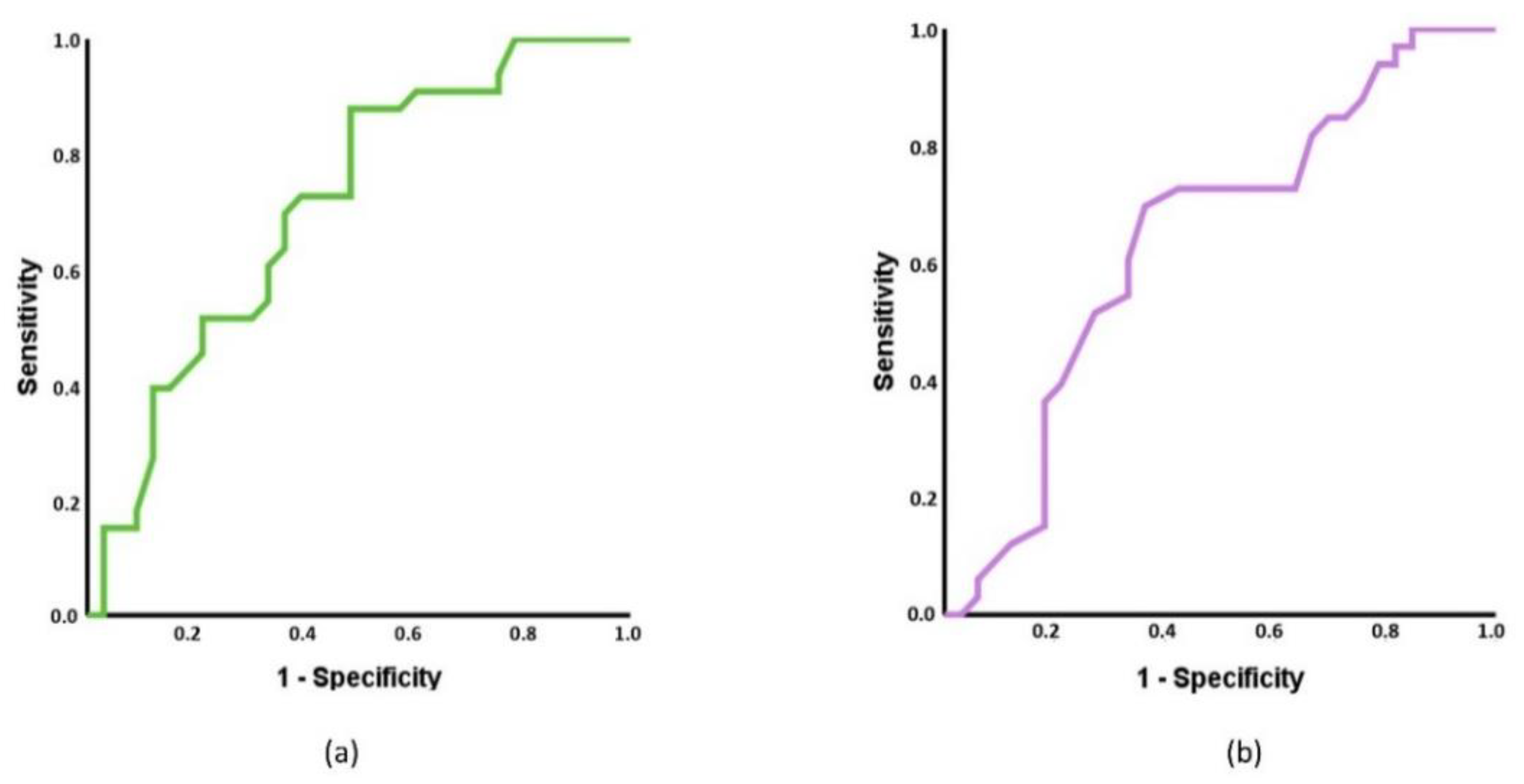
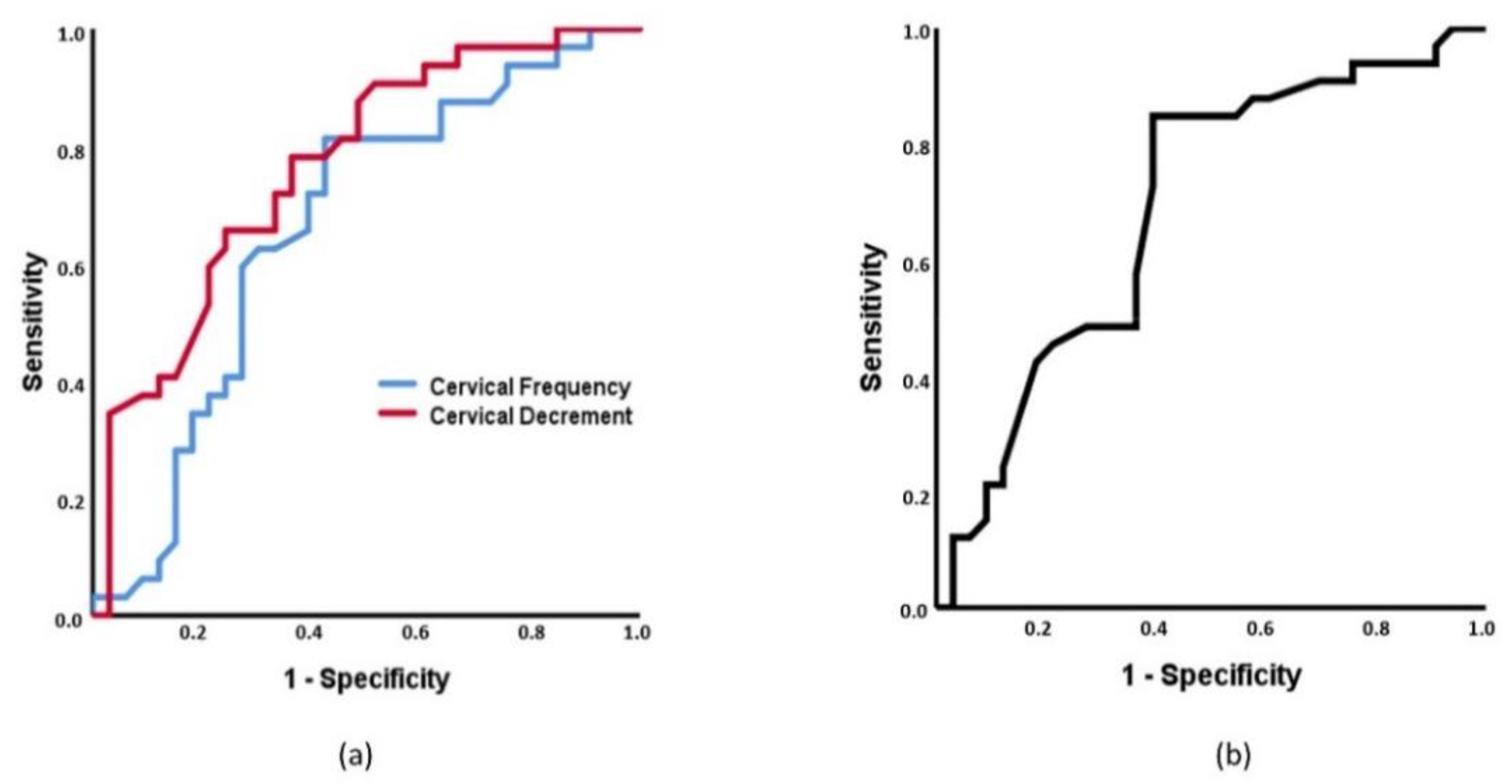
3.2. Receiver Operating Characteristics (ROC) Curves Based on MMPs and ROMs and Multinomial Regression
3.3. Intra-Group Associations among Outcomes, Sociodemographic, and Clinical Variables
4. Discussion
4.1. Differences in MMPs and ROMs between Spinal Pain and Controls
4.2. Capacity of MMPs and ROMs to Discriminate between Spinal Pain and Control Individuals
4.3. Associations between MMPs, ROMs with Sociodemographic and Clinical Features
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| CV | Coefficient of Variation |
| EMG | Electromyography |
| sEMG | surface Electromyography |
| FABQ | Fear-Avoidance Beliefs Questionnaire |
| FABQ-PA | Physical Activity Subscale of Fear-Avoidance Beliefs Questionnaire |
| FABQ-W | Work Subscale of Fear-Avoidance Beliefs Questionnaire |
| ICC | Intraclass Correlation Coefficient |
| IMUs | Inertial Motion Units |
| LBP | Low Back Pain |
| MMP | Muscle Mechanical Property |
| NDI | Neck Disability Index |
| NP | Neck Pain |
| NPRS | Numerical Pain Rating Scale |
| NS | Not Significant |
| NWC | Number of Words Chosen |
| ODI | Oswestry Disability Index |
| OR | Odds Ratio |
| PRI | Pain Rating Index |
| ROC | Receiver Operating Characteristic |
| ROM | Range of Motion |
| SF-12 | 12-item Short-Form Health Survey |
| MCS-12 | Mental Component Summary of 12-item Short-Form Health Survey |
| PCS-12 | Physical Component Summary of 12-item Short-Form Health Survey |
| TSK-11SV | Short Version of Tampa Scale for Kinesiophobia |
| 95%IC | 95% confidence interval |
References
- Clark, S.; Horton, R. Low Back Pain: A Major Global Challenge. Lancet 2018, 391, 2302. [Google Scholar] [CrossRef]
- Abbafati, C.; Machado, D.B.; Cislaghi, B.; Salman, O.M.; Karanikolos, M.; McKee, M.; Abbas, K.M.; Brady, O.J.; Larson, H.J.; Trias-Llimós, S.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- Laird, R.A.; Keating, J.L.; Ussing, K.; Li, P.; Kent, P. Does Movement Matter in People with Back Pain? Investigating “atypical” Lumbo-Pelvic Kinematics in People with and without Back Pain Using Wireless Movement Sensors. BMC Musculoskelet. Disord. 2019, 20, 28. [Google Scholar] [CrossRef]
- Laird, R.A.; Gilbert, J.; Kent, P.; Keating, J.L. Comparing Lumbo-Pelvic Kinematics in People with and without Back Pain: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2014, 15, 229. [Google Scholar] [CrossRef]
- Hides, J.; Gilmore, C.; Stanton, W.; Bohlscheid, E. Multifidus Size and Symmetry among Chronic LBP and Healthy Asymptomatic Subjects. Man. Ther. 2008, 13, 43–49. [Google Scholar] [CrossRef]
- Hildebrandt, M.; Fankhauser, G.; Meichtry, A.; Luomajoki, H. Correlation between Lumbar Dysfunction and Fat Infiltration in Lumbar Multifidus Muscles in Patients with Low Back Pain. BMC Musculoskelet. Disord. 2017, 18, 12. [Google Scholar] [CrossRef]
- Hu, X.; Lei, D.; Li, L.; Leng, Y.; Yu, Q.; Wei, X.; Lo, W.L.A. Quantifying Paraspinal Muscle Tone and Stiffness in Young Adults with Chronic Low Back Pain: A Reliability Study. Sci. Rep. 2018, 8, 14343. [Google Scholar] [CrossRef]
- Kolber, M.J.; Beekhuizen, K. Lumbar Stabilization: An Evidence-Based Approach for the Athlete with Low Back Pain. Strength Cond. J. 2007, 29, 26–37. [Google Scholar] [CrossRef]
- Pranata, A.; Perraton, L.; El-Ansary, D.; Clark, R.; Fortin, K.; Dettmann, T.; Bryant, A. Lumbar Extensor Muscle Force Control Is Associated with Disability in People with Chronic Low Back Pain. Clin. Biomech. 2017, 46, 46–51. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Alex, B.; Csepe, D.; Lancaster, D.; Callaghan, J.P. Altered Muscle Recruitment during Extension from Trunk Flexion in Low Back Pain Developers. Clin. Biomech. 2012, 27, 994–998. [Google Scholar] [CrossRef]
- Cedraschi, C.; Luthy, C.; Allaz, A.F.; Herrmann, F.R.; Ludwig, C. Low Back Pain and Health-Related Quality of Life in Community-Dwelling Older Adults. Eur. Spine J. 2016, 25, 2822–2832. [Google Scholar] [CrossRef]
- Osumi, M.; Sumitani, M.; Otake, Y.; Nishigami, T.; Mibu, A.; Nishi, Y.; Imai, R.; Sato, G.; Nagakura, Y.; Morioka, S. Kinesiophobia Modulates Lumbar Movements in People with Chronic Low Back Pain: A Kinematic Analysis of Lumbar Bending and Returning Movement. Eur. Spine J. 2019, 28, 1572–1578. [Google Scholar] [CrossRef]
- Alhowimel, A.; Alotaibi, M.; Radford, K.; Coulson, N. Psychosocial Factors Associated with Change in Pain and Disability Outcomes in Chronic Low Back Pain Patients Treated by Physiotherapist: A Systematic Review. SAGE Open Med. 2018, 6, 2050312118757387. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; March, L.; Woolf, A.; Blyth, F.; Brooks, P.; Smith, E.; Vos, T.; Barendregt, J.; Blore, J.; Murray, C.; et al. The Global Burden of Neck Pain: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Alsultan, F.; Cescon, C.; Heneghan, N.R.; Rushton, A.; Barbero, M.; Falla, D. Eccentric Exercise and Delayed Onset Muscle Soreness Reduce the Variability of Active Cervical Movements. J. Biomech. 2020, 111, 109962. [Google Scholar] [CrossRef]
- Falla, D.; Farina, D. Neuromuscular Adaptation in Experimental and Clinical Neck Pain. J. Electromyogr. Kinesiol. 2008, 18, 255–261. [Google Scholar] [CrossRef]
- Kocur, P.; Wilski, M.; Lewandowski, J.; Łochyński, D. Female Office Workers with Moderate Neck Pain Have Increased Anterior Positioning of the Cervical Spine and Stiffness of Upper Trapezius Myofascial Tissue in Sitting Posture. PM&R 2018, 11, 476–482. [Google Scholar]
- Meisingset, I.; Stensdotter, A.K.; Woodhouse, A.; Vasseljen, O. Neck Motion, Motor Control, Pain and Disability: A Longitudinal Study of Associations in Neck Pain Patients in Physiotherapy Treatment. Man. Ther. 2016, 22, 94–100. [Google Scholar] [CrossRef]
- Hidalgo, B.; Hall, T.; Bossert, J.; Dugeny, A.; Cagnie, B.; Pitance, L. The Efficacy of Manual Therapy and Exercise for Treating Non-Specific Neck Pain: A Systematic Review. J. Back Musculoskelet. Rehabil. 2017, 30, 1149–1169. [Google Scholar] [CrossRef]
- Dupeyron, A.; Lanhers, C.; Bastide, S.; Alonso, S.; Toulotte, M.; Jourdan, C.; Coudeyre, E. The Back Belief Questionnaire Is Efficient to Assess False Beliefs and Related Fear in Low Back Pain Populations: A Transcultural Adaptation and Validation Study. PLoS ONE 2017, 12, e0186753. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.; Bevers, K.; Licciardone, J.; Su, J.; Du, Y.; Brotto, M. Transitioning from Acute to Chronic Pain: An Examination of Different Trajectories of Low-Back Pain. Healthcare 2018, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Maestre, C.; Esteve, R.; Ruiz-Párraga, G.; Gómez-Pérez, L.; López-Martínez, A.E. The Key Role of Pain Catastrophizing in the Disability of Patients with Acute Back Pain. Int. J. Behav. Med. 2017, 24, 239–248. [Google Scholar] [CrossRef] [PubMed]
- De Vos Andersen, N.B.; Kent, P.; Hjort, J.; Christiansen, D.H. Clinical Course and Prognosis of Musculoskeletal Pain in Patients Referred for Physiotherapy: Does Pain Site Matter? BMC Musculoskelet. Disord. 2017, 18, 130. [Google Scholar] [CrossRef]
- Maissan, F.; Pool, J.; de Raaij, E.; Mollema, J.; Ostelo, R.; Wittink, H. The Clinical Reasoning Process in Randomized Clinical Trials with Patients with Non-Specific Neck Pain Is Incomplete: A Systematic Review. Musculoskelet. Sci. Pract. 2018, 35, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The Global Burden of Low Back Pain: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, A.; Hai, Y.; Li, W.; Yin, L.; Guo, R. Asymmetric Biomechanical Characteristics of the Paravertebral Muscle in Adolescent Idiopathic Scoliosis. Clin. Biomech. 2019, 65, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mustalampi, S.; Ylinen, J.; Korniloff, K.; Weir, A.; Häkkinen, A. Reduced Neck Muscle Strength and Altered Muscle Mechanical Properties in Cervical Dystonia Following Botulinum Neurotoxin Injections: A Prospective Study. J. Mov. Disord. 2016, 9, 44–49. [Google Scholar] [CrossRef]
- Kelly, J.P.; Koppenhaver, S.L.; Michener, L.A.; Proulx, L.; Bisagni, F.; Cleland, J.A. Characterization of Tissue Stiffness of the Infraspinatus, Erector Spinae, and Gastrocnemius Muscle Using Ultrasound Shear Wave Elastography and Superficial Mechanical Deformation. J. Electromyogr. Kinesiol. 2018, 38, 73–80. [Google Scholar] [CrossRef]
- Andonian, B.J.; Masi, A.T.; Aldag, J.C.; Barry, A.J.; Coates, B.A.; Emrich, K.; Henderson, J.; Kelly, J.; Nair, K. Greater Resting Lumbar Extensor Myofascial Stiffness in Younger Ankylosing Spondylitis Patients Than Age-Comparable Healthy Volunteers Quantified by Myotonometry. Arch. Phys. Med. Rehabil. 2015, 96, 2041–2047. [Google Scholar] [CrossRef]
- Ilahi, S.; Alfonse, T.M.; White, A.; Devos, A.; Henderson, J.; Nair, K. Quantified Biomechanical Properties of Lower Lumbar Myofascia in Younger Adults with Chronic Idiopathic Low Back Pain and Matched Healthy Controls. Clin. Biomech. 2020, 73, 78–85. [Google Scholar] [CrossRef]
- Kocur, P.; Wilski, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Influence of Forward Head Posture on Myotonometric Measurements of Superficial Neck Muscle Tone, Elasticity, and Stiffness in Asymptomatic Individuals with Sedentary Jobs. J. Manipulative Physiol. Ther. 2019, 42, 195–202. [Google Scholar] [CrossRef]
- Papi, E.; Bull, A.M.J.; McGregor, A.H. Is There Evidence to Use Kinematic/Kinetic Measures Clinically in Low Back Pain Patients? A Systematic Review. Clin. Biomech. 2018, 55, 53–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Haghighi, P.D.; Burstein, F.; Yap, L.W.; Cheng, W.; Yao, L.; Cicuttini, F. Electronic Skin Wearable Sensors for Detecting Lumbar–Pelvic Movements. Sensors 2020, 20, 1510. [Google Scholar] [CrossRef] [PubMed]
- De Rosario, H.; Vivas, M.J.; Sinovas, M.I.; Page, Á. Relationship between Neck Motion and Self-Reported Pain in Patients with Whiplash Associated Disorders during the Acute Phase. Musculoskelet. Sci. Pract. 2018, 38, 23–29. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Galán-Mercant, A.; Williams, J.M. The Use of Inertial Sensors System for Human Motion Analysis. Phys. Ther. Rev. 2010, 15, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.M.; Heimgartner, M.; Rast, F.M.; Ernst, M.J.; Oetiker, S.; Kool, J. Reliability of Lumbar Movement Dysfunction Tests for Chronic Low Back Pain Patients. Man. Ther. 2016, 24, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Loh, E.; Dickey, J.P.; Walton, D.M.; Trejos, A.L. Development of the Circumduction Metric for Identification of Cervical Motion Impairment. J. Rehabil. Assist. Technol. Eng. 2018, 5, 2055668318777984. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo-Herrera, A.J.; Jové-Blanco, C.; Martínez-Beltrán, M.J.; Moreno-Ruiz, J.A.; de la Torre-Montero, J.C. Improving the Staging of Neck Injuries Using a New Index, the Neck Functional Holistic Analysis Score: Clustering Approach to Determine Degrees of Impairment. PLoS ONE 2020, 15, e0238424. [Google Scholar] [CrossRef]
- Felicio, D.C.; Pereira, D.S.; Diz, J.B.M.; De Queiroz, B.Z.; Da Silva, J.P.; Leopoldino, A.A.O.; Pereira, L.S.M. Anterior Trunk Mobility Does Not Predict Disability in Elderly Women with Acute Low Back Pain: Brazilian Back Complaints in the Elders (BACE-Brazil) Study Results. Spine (Phila Pa 1976) 2017, 42, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef]
- Abbott, J.H.; Schmitt, J. Minimum Important Differences for the Patient-Specific Functional Scale, 4 Region-Specific Outcome Measures, and the Numeric Pain Rating Scale. J. Orthop. Sports Phys. Ther. 2014, 44, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Olivo, S.; Warren, S.; Fuentes, J.; Magee, D.J. Clinical Relevance vs. Statistical Significance: Using Neck Outcomes in Patients with Temporomandibular Disorders as an Example. Man. Ther. 2011, 16, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.; Masi, A.T.; Andonian, B.J.; Barry, A.J.; Coates, B.A.; Dougherty, J.; Schaefer, E.; Henderson, J.; Kelly, J. Stiffness of Resting Lumbar Myofascia in Healthy Young Subjects Quantified Using a Handheld Myotonometer and Concurrently with Surface Electromyography Monitoring. J. Bodyw. Mov. Ther. 2016, 20, 388–396. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Xu, W.; Liang, J.; Guan, Y.; Xu, X. Analysis of Biomechanical Properties of the Lumbar Extensor Myofascia in Elderly Patients with Chronic Low Back Pain and That in Healthy People. BioMed Res. Int. 2020, 2020, 7649157. [Google Scholar] [CrossRef]
- White, A.; Abbott, H.; Masi, A.T.; Henderson, J.; Nair, K. Biomechanical Properties of Low Back Myofascial Tissue in Younger Adult Ankylosing Spondylitis Patients and Matched Healthy Control Subjects. Clin. Biomech. 2018, 57, 67–73. [Google Scholar] [CrossRef]
- Aranda-Valera, I.C.; Cuesta-Vargas, A.; Garrido-Castro, J.L.; Gardiner, P.V.; López-Medina, C.; Machado, P.M.; Condell, J.; Connolly, J.; Williams, J.M.; Muñoz-Esquivel, K.; et al. Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Validation Study in Axial Spondyloarthritis. Diagnostics 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Kim, B.K.; Lee, S.B.; Choi, W.S.; Yeum, D.M. Effects of Central and Unilateral Posteroanterior Mobilization on Cervical Lordosis, Muscle Stiffness and ROM in Patient with Ankylosing Spondylitis: Case Study. J. Phys. Ther. Sci. 2017, 29, 1276–1280. [Google Scholar] [CrossRef]
- Mjøsund, H.L.; Boyle, E.; Kjaer, P.; Mieritz, R.M.; Skallgård, T.; Kent, P. Clinically Acceptable Agreement between the ViMove Wireless Motion Sensor System and the Vicon Motion Capture System When Measuring Lumbar Region Inclination Motion in the Sagittal and Coronal Planes. BMC Musculoskelet. Disord. 2017, 18, 124. [Google Scholar] [CrossRef]
- Lázaro, C.; Caseras, X.; Whizar-Lugo, V.M.; Wenk, R.; Baldioceda, F.; Bernal, R.; Ovalle, A.; Torrubia, R.; Baños, J.E. Psychometric Properties of a Spanish Version of the McGill Pain Questionnaire in Several Spanish-Speaking Countries. Clin. J. Pain 2001, 17, 365–374. [Google Scholar] [CrossRef]
- Masedo, A.I.; Esteve, R. Some Empirical Evidence Regarding the Validity of the Spanish Version of the McGill Pain Questionnaire (MPQ-SV). Pain 2000, 85, 451–456. [Google Scholar] [CrossRef]
- Lázaro, C.; Bosch, F.; Torrubia, R.; Baños, J.-E. The Development of a Spanish Questionnaire for Assessing Pain: Preliminary Data Concerning Reliability and Validity. Eur. J. Psychol. Assess. 1994, 10, 145–151. [Google Scholar]
- Fairbank, J.; Couper, J.; Davies, J.; O’Brien, J.P. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy 1980, 66, 271–273. [Google Scholar]
- Fairbank, J.C.T.; Pynsent, P.B. The Oswestry Disability Index. Spine (Phila. Pa 1976) 2000, 25, 2940–2953. [Google Scholar] [CrossRef]
- Selva-Sevilla, C.; Ferrara, P.; Geronimo-Pardo, M. Psychometric Properties Study of the Oswestry Disability Index in a Spanish Population with Previous Lumbar Disc Surgery: Homogeneity and Validity. Spine (Phila. Pa 1976) 2019, 1–44, 430–437. [Google Scholar] [CrossRef]
- Vernon, H.; Mior, S. The Neck Disability Index: A Study of Reliability and Validity. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Vos, C.J.; Verhagen, A.P.; Koes, B.W. Reliability and Responsiveness of the Dutch Version of the Neck Disability Index in Patients with Acute Neck Pain in General Practice. Eur. Spine J. 2006, 15, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, F.M.; Bagó, J.; Royuela, A.; Seco, J.; Giménez, S.; Muriel, A.; Abraira, V.; Martín, J.L.; Peña, J.L.; Gestoso, M.; et al. Psychometric Characteristics of the Spanish Version of Instruments to Measure Neck Pain Disability. BMC Musculoskelet. Disord. 2008, 9, 42. [Google Scholar] [CrossRef]
- Haik, M.N.; Alburquerque-Sendin, F.; Fernandes, R.A.S.; Kamonseki, D.H.; Almeida, L.A.; Liebano, R.E.; Camargo, P.R. Biopsychosocial Aspects in Individuals with Acute and Chronic Rotator Cu Ff Related Shoulder Pain: Classification Based on a Decision Tree Analysis. Diagnostics 2020, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Waddell, G.; Newton, M.; Henderson, I.; Somerville, D.; Main, C.J. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the Role of Fear-Avoidance Beliefs in Chronic Low Back Pain and Disability. Pain 1993, 52, 157–168. [Google Scholar] [CrossRef]
- Calley, D.Q.; Jackson, S.; Collins, H.; George, S.Z. Identifying Patient Fear-Avoidance Beliefs by Physical Therapists Managing Patients with Low Back Pain. J. Orthop. Sports Phys. Ther. 2010, 40, 774–783. [Google Scholar] [CrossRef]
- Kovacs, F.M.; Muriel, A.; María Medina, J.; Abraira, V.; Castillo Sánchez, M.D.; Olabe Jaúregui, J.; Spanish Back Pain Research Network. Psychometric Characteristics of the Spanish Version of the FAB Questionnaire. Spine (Phila. Pa 1976) 2006, 31, 104–110. [Google Scholar] [CrossRef]
- Staerkle, R.; Mannion, A.F.; Elfering, A.; Junge, A.; Semmer, N.K.; Jacobshagen, N.; Grob, D.; Dvorak, J.; Boos, N. Longitudinal Validation of the Fear-Avoidance Beliefs Questionnaire (FABQ) in a Swiss-German Sample of Low Back Pain Patients. Eur. Spine J. 2004, 13, 332–340. [Google Scholar] [CrossRef]
- Monticone, M.; Ambrosini, E.; Rocca, B.; Foti, C.; Ferrante, S. Responsiveness and Minimal Clinically Important Changes for the Tampa Scale of Kinesiophobia after Lumbar Fusion during Cognitive Behavioral Rehabilitation. Eur. J. Phys. Rehabil. Med. 2017, 53, 351–358. [Google Scholar] [PubMed]
- Miller, R.P.; Kori, S.; Todd, D. The Tampa Scale: A Measure of Kinesiophobia. Clin. J. Pain 1991, 7, 51–52. [Google Scholar] [CrossRef]
- Gómez-Pérez, L.; López-Martínez, A.E.; Ruiz-Párraga, G.T. Psychometric Properties of the Spanish Version of the Tampa Scale for Kinesiophobia (TSK). J. Pain 2011, 12, 425–435. [Google Scholar] [CrossRef]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-Validation of Item Selection and Scoring for the SF-12 Health Survey in Nine Countries: Results from the IQOLA Project. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Resnick, B.; Parker, B. Simplified Scoring and Psychometrics of the Revised 12-Item Short-Form Health Survey. Nurs. Lond. 2001, 5, 161–166. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. (Eds.) Assessing the Fit of the Model. In Applied Logistic Regression; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 153–226. [Google Scholar]
- Akoglu, H. User’s Guide to Correlation Coefficients. Tur. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Lo, W.L.A.; Yu, Q.; Mao, Y.; Li, W.; Hu, C.; Li, L. Lumbar Muscles Biomechanical Characteristics in Young People with Chronic Spinal Pain. BMC Musculoskelet. Disord. 2019, 20, 559. [Google Scholar] [CrossRef]
- Laird, R.A.; Kent, P.; Keating, J.L. How Consistent Are Lordosis, Range of Movement and Lumbo-Pelvic Rhythm in People with and without Back Pain? BMC Musculoskelet. Disord. 2016, 17, 403. [Google Scholar] [CrossRef]
- Ha, T.H.; Saber-Sheikh, K.; Moore, A.P.; Jones, M.P. Measurement of Lumbar Spine Range of Movement and Coupled Motion Using Inertial Sensors—A Protocol Validity Study. Man. Ther. 2013, 18, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Theobald, P.S.; Jones, M.D.; Williams, J.M. Do Inertial Sensors Represent a Viable Method to Reliably Measure Cervical Spine Range of Motion? Man. Ther. 2012, 17, 92–96. [Google Scholar] [CrossRef]
- Sueki, D.G.; Cleland, J.A.; Wainner, R.S. A Regional Interdependence Model of Musculoskeletal Dysfunction: Research, Mechanisms, and Clinical Implications. J. Man. Manip. Ther. 2013, 21, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Wainner, R.S.; Whitman, J.M.; Cleland, J.A.; Flynn, T.W. Regional Interdependence: A Musculoskeletal Examination Model Whose Time Has Come. J. Orthop. Sports Phys. Ther. 2007, 37, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.R.; Wei, T. Sen Disc Height and Lumbar Index as Independent Predictors of Degenerative Spondylolisthesis in Middle-Aged Women with Low Back Pain. Spine (Phila Pa 1976) 2009, 34, 1402–1409. [Google Scholar] [CrossRef]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Stokes, M. Measurement of Ageing Effects on Muscle Tone and Mechanical Properties of Rectus Femoris and Biceps Brachii in Healthy Males and Females Using a Novel Hand-Held Myometric Device. Arch. Gerontol. Geriatr. 2016, 62, 59–67. [Google Scholar] [CrossRef]
- Fielding, R.; Vellas, B.; Evans, W. Sarcopenia: An Undiagnosed Condition in Older Adults. Consensus Definition: Prevalence, Etiology, and Consequences. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Resnik, L.; Dobrykowski, E. Outcomes Measurement for Patients with Low Back Pain. Orthop. Nurs. 2005, 24, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Maher, R.M.; Hayes, D.M.; Shinohara, M. Quantification of Dry Needling and Posture Effects on Myofascial Trigger Points Using Ultrasound Shear-Wave Elastography. Arch. Phys. Med. Rehabil. 2013, 94, 2146–2150. [Google Scholar] [CrossRef]
- Ritvanen, T.; Zaproudina, N.; Nissen, M.; Leinonen, V.; Hanninen, O. Dynamic Surface Electromyographic Responses in Chronic Low Back Pain Treated by Traditional Bone Setting and Conventional Physical Therapy. J. Manip. Physiol. Ther. 2007, 30, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Murillo, C.; Martinez-Valdes, E.; Heneghan, N.R.; Liew, B.; Rushton, A.; Sanderson, A.; Falla, D. High-Density Electromyography Provides New Insights into the Flexion Relaxation Phenomenon in Individuals with Low Back Pain. Sci. Rep. 2019, 9, 15938. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, K.; Lim, S. Aged Lumbar Extension Strength of Chronic Low Back Pain in Korean Population of 1-Years. Iran. J. Public Health 2020, 49, 1894–1901. [Google Scholar] [PubMed]

| Variables | Low Back Pain (n = 33) | Neck Pain (n = 33) | Healthy (n = 33) | p-Value |
|---|---|---|---|---|
| Age (years) | 41.9 ± 14.8 | 38.8 ± 11.1 | 37.0 ± 10.9 | 0.373 |
| Sex (female/male) | 11/22 | 14/19 | 13/20 | 0.742 |
| BMI (Kg/m2) | 25.9 ± 3.9 | 25.2 ± 4.7 | 23.8 ± 3.5 | 0.160 |
| PCS-12 | 41.6 ± 8.6 | 42.5 ± 9.9 | 54.1 ± 3.7 | <0.001 ‡ |
| MCS-12 | 50.9 ± 9.5 | 50.8 ± 10.4 | 53.1 ± 6.4 | 0.484 |
| NPRS | 4.7 ± 1.6 | 5.4 ± 1.9 | - | 0.100 |
| NWC | 9.3 ± 4.9 | 9.1 ± 4.5 | - | 0.855 |
| PRI-total | 17.1 ± 9.5 | 18.4 ± 9.0 | - | 0.561 |
| NDI | - | 11.0 ± 5.2 | - | |
| ODI | 21.1 ± 12.8 | - | - | |
| FABQ | 31.4 ± 13.6 | 36.2 ± 20.7 | - | 0.283 |
| FABQ-PA | 12.3 ± 6.0 | 12.6 ± 6.7 | - | 0.815 |
| FABQ-W | 11.8 ± 7.4 | 16.1 ± 10.7 | - | 0.072 |
| TSK-11SV | 22.9 ± 6.5 | 22.7 ± 5.9 | - | 0.891 |
| Muscle Mechanical Properties (MMPs) | ||||
| Lumbar tone (Hz) | 14.94 ± 2.54 | 14.70 ± 1.63 | 15.16 ± 2.22 | 0.697 |
| Lumbar stiffness (N/m) | 289.89 ± 76.23 | 279.95 ± 69.16 | 283.72 ± 75.37 | 0.847 |
| Lumbar decrement | 1.41 ± 0.37 | 1.28 ± 0.35 | 1.26 ± 0.32 | 0.169 |
| Lumbar relaxation (ms) | 19.45 ± 4.59 | 19.53 ± 4.24 | 19.48 ± 4.62 | 0.998 |
| Lumbar creep (Deborah number) | 1.21 ± 0.27 | 1.15 ± 0.23 | 1.21 ± 0.24 | 0.519 |
| Cervical tone (Hz) | 15.86 ± 2.09 | 16.52 ± 1.78 | 15.42 ± 2.24 | 0.048 § |
| Cervical stiffness (N/m) | 275.92 ± 57.19 | 290.43 ± 53.97 | 265.14 ± 72.17 | 0.258 |
| Cervical decrement | 1.43 ± 0.22 | 1.45 ± 0.18 | 1.27 ± 0.23 | 0.001 ‡ |
| Cervical relaxation (ms) | 19.62 ± 3.87 | 18.10 ± 2.48 | 19.17 ± 4.08 | 0.214 |
| Cervical creep (Deborah number) | 1.17 ± 0.20 | 1.11 ± 0.13 | 1.15 ± 0.20 | 0.312 |
| Spinal Mobility (Range of Motion, ROM) | ||||
| Lumbar flexion (°) | 49.0 ± 14.3 | 57.1 ± 12.6 | 53.8 ± 9.4 | 0.031 * |
| Lumbar extension (°) | 18.6 ± 18.2 | 17.2 ± 10.5 | 16.9 ± 11.9 | 0.871 |
| Lumbar rotation (°) | 27.2 ± 11.8 | 31.0 ± 10.0 | 27.9 ± 8.5 | 0.279 |
| Lumbar lateral-flexion (°) | 54.7 ± 13.5 | 55.5 ± 8.8 | 56.5 ± 9.8 | 0.807 |
| Cervical flexion (°) | 51.8 ± 8.8 | 46.0 ± 9.9 | 51.8 ± 7.4 | 0.010 † |
| Cervical extension (°) | 45.4 ± 11.7 | 45.2 ± 16.1 | 50.5 ± 13.0 | 0.209 |
| Cervical rotation (°) | 135.3 ± 19.5 | 127.8 ± 25.2 | 139.9 ± 15.0 | 0.047 § |
| Cervical lateral-flexion (°) | 73.9 ± 19.6 | 68.6 ± 17.2 | 69.6 ± 11.4 | 0.383 |
| State | Group | Estimated State | |||
|---|---|---|---|---|---|
| Low Back Pain | Neck Pain | Control | Percentage of Correct Estimation | ||
| Real state | Low Back Pain | 19 | 6 | 8 | 57.6% |
| Neck Pain | 7 | 23 | 2 | 71.9% | |
| Control | 5 | 4 | 24 | 72.7% | |
| Global percentage | 31.6% | 33.7% | 34.7% | 67.3% | |
| Variables | Lumbar Tone | Lumbar Stiffness | Lumbar Decrement | Lumbar Relaxation | Lumbar Creep | Cervical Tone | Cervical Stiffness | Cervical Decrement | Cervical Relaxation | Cervical Creep | Lumbar Flexion | Lumbar Extension | Lumbar Rotation | Lumbar Lateral-Flexion | Cervical Flexion | Cervical Extension | Cervical Rotation | Cervical Lateral-Flexion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.554 | 0.555 | 0.565 | −0.455 | NS | 0.342 | NS | 0.687 | NS | NS | −0.547 | NS | NS | −0.397 | −0.545 | −0.460 | −0.352 | −0.533 |
| BMI | NS | NS | NS | NS | NS | −0.416 | −0.436 | NS | 0.468 | 0.455 | NS | NS | NS | NS | NS | −0.421 | NS | −0.400 |
| Lumbar flexion | −0.372 | −0.359 | NS | NS | NS | NS | NS | −0.399 | NS | NS | ||||||||
| Lumbar extension | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Lumbar rotation | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Lumbar lateral-flexion | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Cervical flexion | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Cervical extension | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Cervical rotation | −0.529 | −0.534 | −0.386 | 0.405 | 0.352 | NS | NS | NS | NS | NS | ||||||||
| Cervical lateral-flexion | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Variables | Lumbar Tone | Lumbar Stiffness | Lumbar Decrement | Lumbar Relaxation | Lumbar Creep | Cervical Tone | Cervical Stiffness | Cervical Decrement | Cervical Relaxation | Cervical Creep | Lumbar Flexion | Lumbar Extension | Lumbar Rotation | Lumbar Lateral-Flexion | Cervical Flexion | Cervical Extension | Cervical Rotation | Cervical Lateral-Flexion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | NS | NS | 0.458 | NS | NS | NS | NS | 0.541 | NS | NS | −0.459 | NS | NS | −0.401 | NS | −0.512 | −0.483 | −0.674 |
| BMI | NS | NS | NS | 0.351 | 0.379 | NS | NS | NS | NS | 0.341 | −0.388 | NS | NS | −0.423 | −0.403 | −0.513 | NS | NS |
| Lumbar flexion | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Lumbar extension | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Lumbar rotation | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Lumbar lateral-flexion | NS | NS | −0.545 | NS | −0.348 | NS | NS | NS | NS | NS | ||||||||
| Cervical flexion | NS | NS | NS | NS | −0.409 | NS | NS | NS | NS | NS | ||||||||
| Cervical extension | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Cervical rotation | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Cervical lateral-flexion | NS | NS | −0.522 | NS | NS | NS | NS | −0.364 | NS | NS |
| Variables | Lumbar Tone | Lumbar Stiffness | Lumbar Decrement | Lumbar Relaxation | Lumbar Creep | Cervical Tone | Cervical Stiffness | Cervical Decrement | Cervical Relaxation | Cervical Creep | Lumbar Flexion | Lumbar Extension | Lumbar Rotation | Lumbar Lateral-Flexion | Cervical Flexion | Cervical Extension | Cervical Rotation | Cervical Lateral-Flexion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.644 | 0.598 | 0.653 | −0.629 | −0.410 | NS | NS | 0.529 | NS | NS | −0.376 | −0.650 | NS | NS | NS | −0.354 | NS | NS |
| BMI | NS | NS | NS | NS | NS | NS | NS | NS | 0.494 | 0.510 | −0.367 | NS | NS | −0.459 | NS | −0.502 | NS | NS |
| Lumbar flexion | −0.411 | −0.341 | −0.384 | 0.413 | 0.341 | NS | NS | NS | NS | NS | ||||||||
| Lumbar extension | −0.354 | −0.381 | −0.490 | 0.629 | 0.570 | NS | NS | −0.401 | NS | NS | ||||||||
| Lumbar rotation | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Lumbar lateral-flexion | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Cervical flexion | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Cervical extension | NS | NS | NS | NS | NS | NS | NS | NS | NS | −0.352 | ||||||||
| Cervical rotation | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Cervical lateral-flexion | NS | NS | −0.464 | NS | NS | NS | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz-Clariana, S.; García-Luque, L.; Garrido-Castro, J.L.; Fernández-de-las-Peñas, C.; Carmona-Pérez, C.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendín, F. Paravertebral Muscle Mechanical Properties and Spinal Range of Motion in Patients with Acute Neck or Low Back Pain: A Case-Control Study. Diagnostics 2021, 11, 352. https://doi.org/10.3390/diagnostics11020352
Alcaraz-Clariana S, García-Luque L, Garrido-Castro JL, Fernández-de-las-Peñas C, Carmona-Pérez C, Rodrigues-de-Souza DP, Alburquerque-Sendín F. Paravertebral Muscle Mechanical Properties and Spinal Range of Motion in Patients with Acute Neck or Low Back Pain: A Case-Control Study. Diagnostics. 2021; 11(2):352. https://doi.org/10.3390/diagnostics11020352
Chicago/Turabian StyleAlcaraz-Clariana, Sandra, Lourdes García-Luque, Juan Luis Garrido-Castro, César Fernández-de-las-Peñas, Cristina Carmona-Pérez, Daiana Priscila Rodrigues-de-Souza, and Francisco Alburquerque-Sendín. 2021. "Paravertebral Muscle Mechanical Properties and Spinal Range of Motion in Patients with Acute Neck or Low Back Pain: A Case-Control Study" Diagnostics 11, no. 2: 352. https://doi.org/10.3390/diagnostics11020352
APA StyleAlcaraz-Clariana, S., García-Luque, L., Garrido-Castro, J. L., Fernández-de-las-Peñas, C., Carmona-Pérez, C., Rodrigues-de-Souza, D. P., & Alburquerque-Sendín, F. (2021). Paravertebral Muscle Mechanical Properties and Spinal Range of Motion in Patients with Acute Neck or Low Back Pain: A Case-Control Study. Diagnostics, 11(2), 352. https://doi.org/10.3390/diagnostics11020352









