Initial Experience with 64Cu-DOTATATE Digital PET of Patients with Neuroendocrine Neoplasms: Comparison with Analog PET
Abstract
1. Introduction
2. Materials and Methods
2.1. Image Acquisition and Analysis
2.2. Statistical Analysis
3. Results
3.1. Patients and PET Acquisition Characteristics
3.2. Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezaei, S.; Ghafarian, P.; Bakhshayesh-Karam, M.; Uribe, C.F.; Rahmim, A.; Sarkar, S.; Ay, M.R. The impact of iterative reconstruction protocol, signal-to-background ratio and background activity on measurement of PET spatial resolution. Jpn. J. Radiol. 2020, 38, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kunnen, B.; Beijst, C.; Lam, M.; Viergever, M.A.; de Jong, H. Comparison of the Biograph Vision and Biograph mCT for quantitative (90)Y PET/CT imaging for radioembolisation. EJNMMI Phys. 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, G.; Ishikawa, K.; Mitsumoto, K.; Taniguchi, T.; Ohya, N.; Baba, S.; Abe, K.; Sasaki, M. Improvement in PET/CT image quality with a combination of point-spread function and time-of-flight in relation to reconstruction parameters. J. Nucl. Med. 2012, 53, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, J.; de Jong, J.; Schaar, J.; Noordzij, W.; van Snick, P.; Dierckx, R.; Borra, R.; Willemsen, A.; Boellaard, R. Performance Characteristics of the Digital Biograph Vision PET/CT System. J. Nucl. Med. 2019, 60, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Gnesin, S.; Kieffer, C.; Zeimpekis, K.; Papazyan, J.P.; Guignard, R.; Prior, J.O.; Verdun, F.R.; Lima, T.V.M. Phantom-based image quality assessment of clinical (18)F-FDG protocols in digital PET/CT and comparison to conventional PMT-based PET/CT. EJNMMI Phys. 2020, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, J.; Boellaard, R.; Dierckx, R.; Stormezand, G.N.; Glaudemans, A.; Noordzij, W. Image Quality and Activity Optimization in Oncologic (18)F-FDG PET Using the Digital Biograph Vision PET/CT System. J. Nucl. Med. 2020, 61, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Halfdanarson, T.R.; Bellizzi, A.M.; Chan, J.A.; Dillon, J.S.; Heaney, A.P.; Kunz, P.L.; O’Dorisio, T.M.; Salem, R.; Segelov, E.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas 2017, 46, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Janson, E.T.; Sorbye, H.; Welin, S.; Federspiel, B.; Gronbaek, H.; Hellman, P.; Ladekarl, M.; Langer, S.W.; Mortensen, J.; Schalin-Jantti, C.; et al. Nordic guidelines 2014 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. 2014, 53, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Johnbeck, C.B.; Knigge, U.; Kjaer, A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: Current status and review of the literature. Future Oncol. 2014, 10, 2259–2277. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Knigge, U.; Mortensen, J.; Oturai, P.; Berthelsen, A.K.; Loft, A.; Binderup, T.; Rasmussen, P.; Elema, D.; Klausen, T.L.; et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: First-in-humans study. J. Nucl. Med. 2012, 53, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Knigge, U.; Binderup, T.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Rasmussen, P.; Elema, D.; et al. 64Cu-DOTATATE PET for Neuroendocrine Tumors: A Prospective Head-to-Head Comparison with 111In-DTPA-Octreotide in 112 Patients. J. Nucl. Med. 2015, 56, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-Head Comparison of (64)Cu-DOTATATE and (68)Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Loft, M.; Carlsen, E.A.; Johnbeck, C.B.; Johannesen, H.H.; Binderup, T.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; et al. (64)Cu-DOTATATE PET in Patients with Neuroendocrine Neoplasms: Prospective, Head-to-Head Comparison of Imaging at 1 Hour and 3 Hours After Injection. J. Nucl. Med. 2021, 62, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.A.; Johnbeck, C.B.; Binderup, T.; Loft, M.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; et al. (64)Cu-DOTATATE PET/CT and Prediction of Overall and Progression-Free Survival in Patients with Neuroendocrine Neoplasms. J. Nucl. Med. 2020, 61, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, J.; Boellaard, R.; Somasundaram, A.; van Snick, P.H.; Borra, R.J.H.; Dierckx, R.; Stormezand, G.N.; Glaudemans, A.; Noordzij, W. Image Quality and Semiquantitative Measurements on the Biograph Vision PET/CT System: Initial Experiences and Comparison with the Biograph mCT. J. Nucl. Med. 2020, 61, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Alberts, I.; Prenosil, G.; Sachpekidis, C.; Weitzel, T.; Shi, K.; Rominger, A.; Afshar-Oromieh, A. Digital versus analogue PET in [(68)Ga]Ga-PSMA-11 PET/CT for recurrent prostate cancer: A matched-pair comparison. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef] [PubMed]



| Analog PET | Digital PET | |||||
|---|---|---|---|---|---|---|
| Pt | Dose (MBq) | Accumulation Time (min) | Acquisition Time (min:s) | Dose (MBq) | Accumulation Time (min) | Acquisition Time (min:s) |
| 1 | 182 | 71 | 24:39 | 182 | 81 | 07:59 |
| 2 | 198 | 80 | 24:39 | 201 | 57 | 09:27 |
| 3 | 209 | 78 | 24:39 | 188 | 62 | 08:10 |
| 4 | 189 | 83 | 28:44 | 196 | 83 | 09:45 |
| 5 | 190 | 62 | 24:39 | 194 | 63 | 06:17 |
| Mean | 194 | 75 | 25:28 | 192 | 69 | 08:20 |
| Pt | Treatment at Analog PET/CT | Changes between Analog and Digital PET/CT |
|---|---|---|
| 1 | None | Lanreotide, 120 mg/4 weeks |
| 2 | Octreotide, 40 mg/3 weeks + Interferon-α, 3 MIU/3 weeks | Stopped interferon-α |
| 3 | Everolimus, 10 mg/day | No |
| 4 | Lanreotide, 120 mg/4 weeks | No |
| 5 | None | Lanreotide, 120 mg/4 weeks + streptozotocin/5FU |
| Pt | T 1 | Analog PET/CT 2 | Digital PET/CT 2 | Lesion Changes 3 | Figure | Notes/Follow-Up | ||
|---|---|---|---|---|---|---|---|---|
| PET | CT | PET | CT | |||||
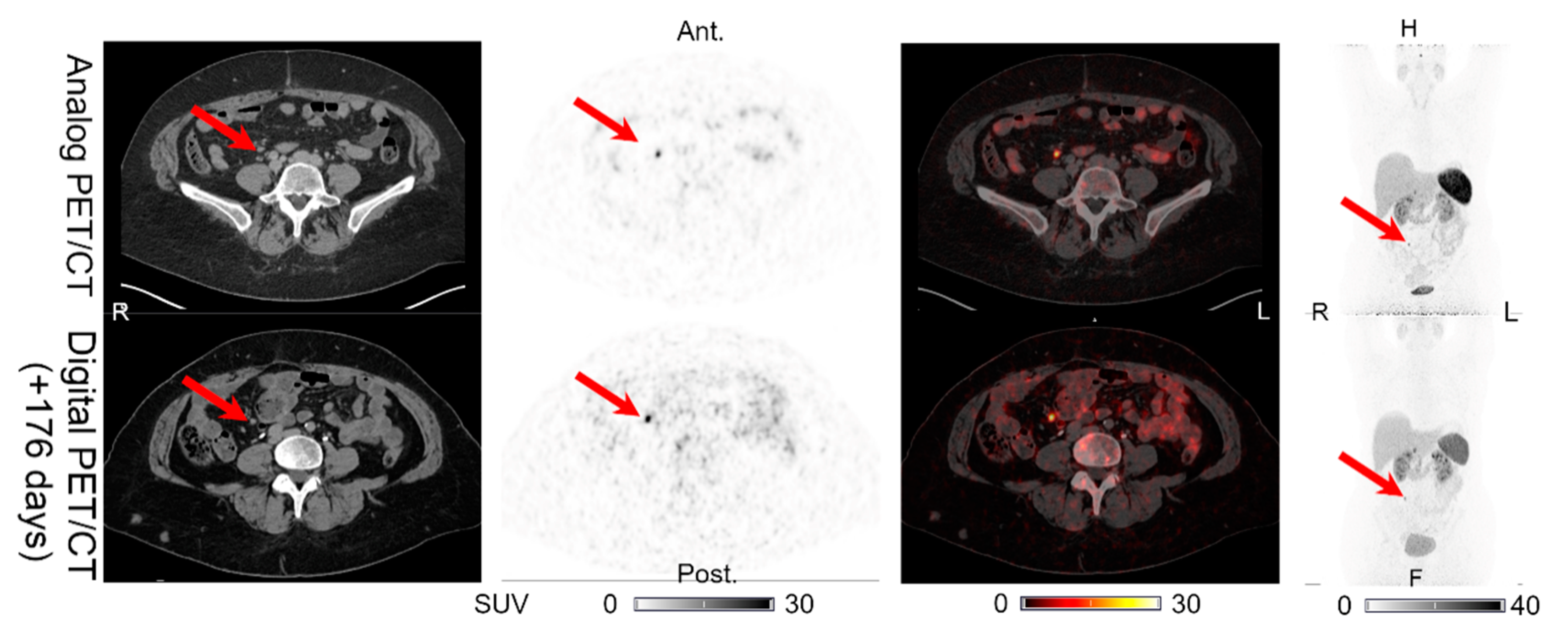
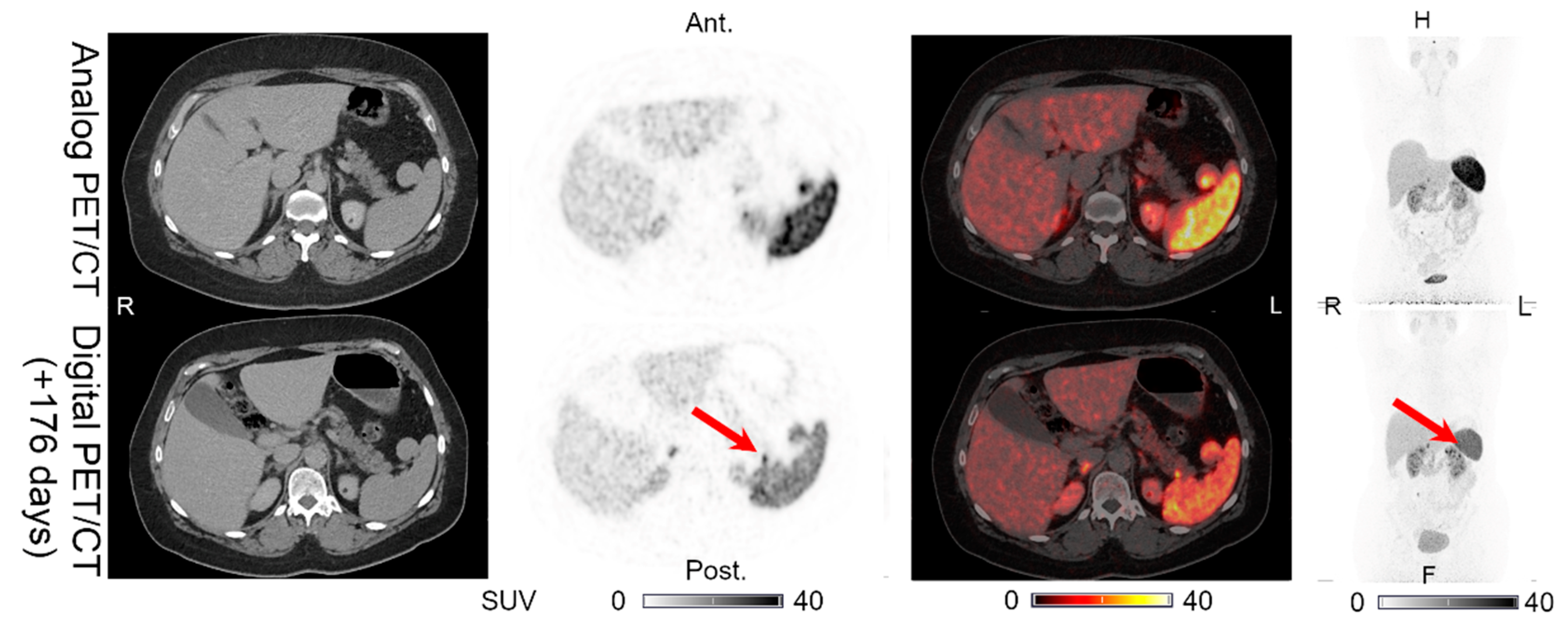
| 1 | 176 | LN (1) | LN (1) | LN (1) | LN (1) | UNCH | Figure 1 | No IV contrast on CT of digital PET/CT. CT with IV contrast performed 189 days after the digital PET/CT shows normal pancreas. |
| PAN (0) | PAN (0) | PAN (1) | PAN (0) | PET (+)/CT (0) | Figure 6 | |||
| 2 | 120 | HEP (>15) | HEP (>15) | HEP (>15) | HEP (>15) | UNCH | Figure 2a | |
| OSS (6) | OSS (2) | OSS (6) | OSS (2) | UNCH | - | |||
| PAN (1) | PAN (1) | PAN (1) | PAN (1) | UNCH | Figure 2b | |||
| 3 | 107 | HEP (5) | HEP (5) | HEP (5) | HEP (5) | UNCH | Figure 3a | |
| LN (2) | LN (2) | LN (2) | LN (2) | UNCH | Figure 3b | |||
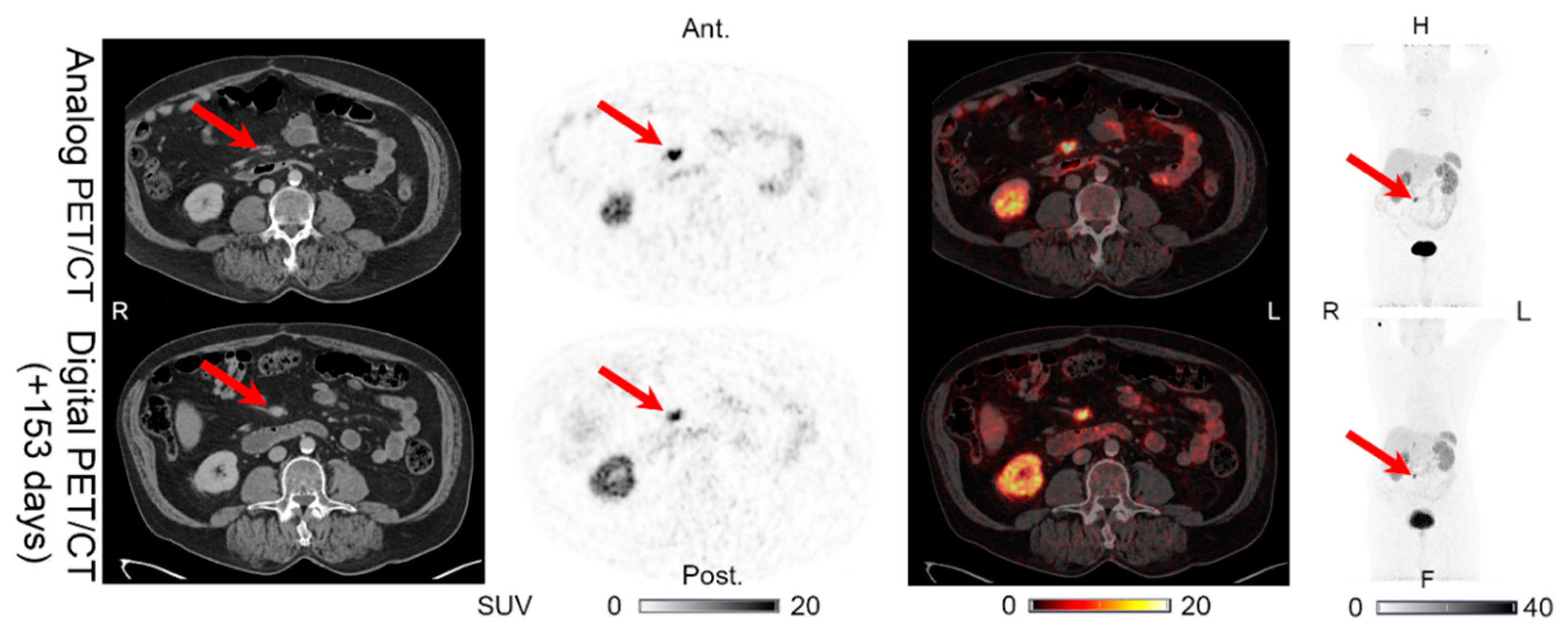
| 4 | 153 | INT (1) | INT (1) | INT (1) | INT (1) | UNCH | Figure 4 | Sternal fracture visible on the analog PET/CT. |
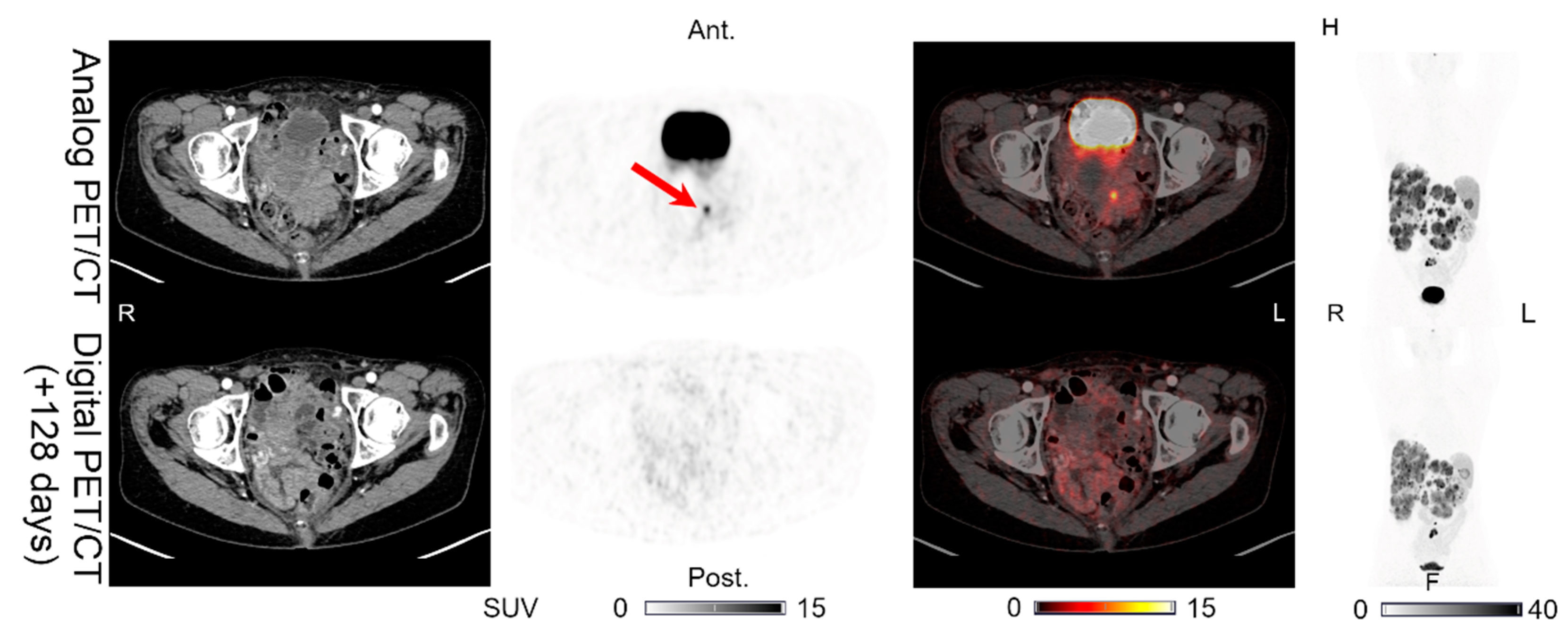
| 5 | 128 | HEP (>15) | HEP (>15) | HEP (>15) | HEP (>15) | UNCH | - | CT with IV contrast performed 89 days after the digital PET/CT shows no new intestinal lesions. No other SSTR-PET imaging available. |
| INT (1) | INT (0) | INT (0) | INT (0) | PET (÷)/CT (0) | Figure 7 | |||
| LN (1) | LN (1) | LN (1) | LN (1) | UNCH | - | |||
| OSS (1) | OSS (1) | OSS (1) | OSS (1) | UNCH | Figure 5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loft, M.; Johnbeck, C.B.; Carlsen, E.A.; Johannesen, H.H.; Oturai, P.; Langer, S.W.; Knigge, U.; Kjaer, A. Initial Experience with 64Cu-DOTATATE Digital PET of Patients with Neuroendocrine Neoplasms: Comparison with Analog PET. Diagnostics 2021, 11, 350. https://doi.org/10.3390/diagnostics11020350
Loft M, Johnbeck CB, Carlsen EA, Johannesen HH, Oturai P, Langer SW, Knigge U, Kjaer A. Initial Experience with 64Cu-DOTATATE Digital PET of Patients with Neuroendocrine Neoplasms: Comparison with Analog PET. Diagnostics. 2021; 11(2):350. https://doi.org/10.3390/diagnostics11020350
Chicago/Turabian StyleLoft, Mathias, Camilla B. Johnbeck, Esben A. Carlsen, Helle H. Johannesen, Peter Oturai, Seppo W. Langer, Ulrich Knigge, and Andreas Kjaer. 2021. "Initial Experience with 64Cu-DOTATATE Digital PET of Patients with Neuroendocrine Neoplasms: Comparison with Analog PET" Diagnostics 11, no. 2: 350. https://doi.org/10.3390/diagnostics11020350
APA StyleLoft, M., Johnbeck, C. B., Carlsen, E. A., Johannesen, H. H., Oturai, P., Langer, S. W., Knigge, U., & Kjaer, A. (2021). Initial Experience with 64Cu-DOTATATE Digital PET of Patients with Neuroendocrine Neoplasms: Comparison with Analog PET. Diagnostics, 11(2), 350. https://doi.org/10.3390/diagnostics11020350









