A SNaPshot Assay for Determination of the Mannose-Binding Lectin Gene Variants and an Algorithm for Calculation of Haplogenotype Combinations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Sampling
2.2. DNA Isolation
2.3. SNaPshot Assay
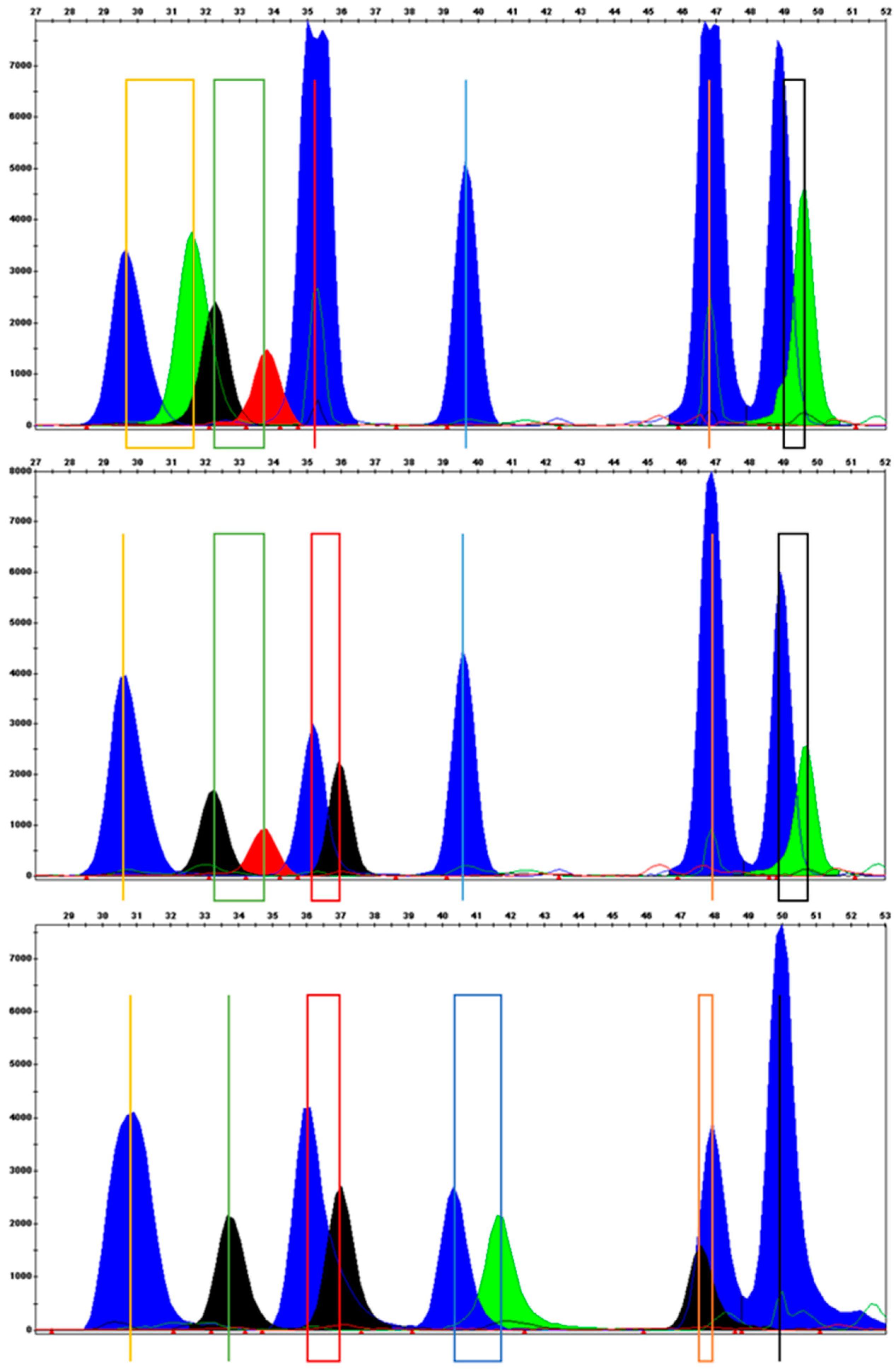
2.3.1. Primer Design
2.3.2. Amplification PCR
2.3.3. Single-Base Extension (SBE) Reaction
2.3.4. Capillary Electrophoresis
2.4. Sanger Sequencing
2.5. Calculation of Haplogenotype Combinations
2.6. Statistical Analysis
3. Results
3.1. Design and Optimization of the SNaPshot Assay
3.2. Mannose-Binding Lectin Gene (MBL2) Genotyping
3.3. Validation of the SNaPshot Assay Results
3.4. Calculation of Haplogenotype Combinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dommett, R.M.; Klein, N.; Turner, M.W. Mannose-Binding Lectin in Innate Immunity: Past, Present and Future. Tissue Antigens 2006, 68, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ip, W.E.; Michelow, I.C.; Ezekowitz, R.A.B. The Mannose-Binding Lectin: A Prototypic Pattern Recognition Molecule. Curr. Opin. Immunol. 2006, 18, 16–23. [Google Scholar] [CrossRef]
- Ip, E.W.K.; Takahashi, K.; Ezekowitz, A.R.B.; Stuart, L.M. Mannose-Binding Lectin and Innate Immunity. Immunol. Rev. 2009, 230, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cedzyński, M.; Kilpatrick, D.C.; Świerzko, A.S. Mannose-Binding Lectin. In The Complement FactsBook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 33–43. ISBN 978-0-12-810420-0. [Google Scholar]
- dos Santos Silva, P.M.; de Oliveira, W.F.; Albuquerque, P.B.S.; dos Santos Correia, M.T.; Coelho, L.C.B.B. Insights into Anti-Pathogenic Activities of Mannose Lectins. Int. J. Biol. Macromol. 2019, 140, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, T.R.; Thiel, S.; Andersen, G.R. Toward a Structure-Based Comprehension of the Lectin Pathway of Complement. Mol. Immunol. 2013, 56, 222–231. [Google Scholar] [CrossRef]
- Kjaer, T.R.; Jensen, L.; Hansen, A.; Dani, R.; Jensenius, J.C.; Dobó, J.; Gál, P.; Thiel, S. Oligomerization of Mannan-Binding Lectin Dictates Binding Properties and Complement Activation. Scand. J. Immunol. 2016, 84, 12–19. [Google Scholar] [CrossRef]
- Garred, P.; Genster, N.; Pilely, K.; Bayarri-Olmos, R.; Rosbjerg, A.; Ma, Y.J.; Skjoedt, M.-O. A Journey through the Lectin Pathway of Complement-MBL and Beyond. Immunol. Rev. 2016, 274, 74–97. [Google Scholar] [CrossRef]
- Dobó, J.; Kocsis, A.; Gál, P. Be on Target: Strategies of Targeting Alternative and Lectin Pathway Components in Complement-Mediated Diseases. Front. Immunol. 2018, 9, 1851. [Google Scholar] [CrossRef]
- Heitzeneder, S.; Seidel, M.; Förster-Waldl, E.; Heitger, A. Mannan-Binding Lectin Deficiency—Good News, Bad News, Doesn’t Matter? Clin. Immunol. 2012, 143, 22–38. [Google Scholar] [CrossRef]
- Verdu, P.; Barreiro, L.B.; Patin, E.; Gessain, A.; Cassar, O.; Kidd, J.R.; Kidd, K.K.; Behar, D.M.; Froment, A.; Heyer, E.; et al. Evolutionary Insights into the High Worldwide Prevalence of MBL2 Deficiency Alleles. Hum. Mol. Genet. 2006, 15, 2650–2658. [Google Scholar] [CrossRef]
- Seyfarth, J.; Garred, P.; Madsen, H.O. The ‘Involution’ of Mannose-Binding Lectin. Hum. Mol. Genet. 2005, 14, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, L.H.; Roep, B.O.; Roos, A. Mannose-Binding Lectin: Clinical Implications for Infection, Transplantation, and Autoimmunity. Hum. Immunol. 2006, 67, 247–256. [Google Scholar] [CrossRef]
- Hou, J.; Ding, J.; Li, L.; Peng, Y.; Gao, X.; Guo, Z. Association of Sirtuin 1 Gene Polymorphisms with Nephrolithiasis in Eastern Chinese Population. Ren. Fail. 2019, 41, 34–41. [Google Scholar] [CrossRef]
- Geng, Y.; Li, L.; Wang, X.; He, F.; Zhou, Y.; Yang, M.; Xu, Y. Interleukin-10 Polymorphisms Affect the Key Periodontal Pathogens in Chinese Periodontitis Patients. Sci. Rep. 2018, 8, 9068. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Liu, Y.; Sun, G.; Tian, H.; Hong, L. Polymorphisms in MAPK9 (Rs4147385) and CSF1R (Rs17725712) Are Associated with the Development of Inhibitors in Patients with Haemophilia A in North China. Int. J. Lab. Hematol. 2019, 41, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.; Maniatis, T. Molecular Cloning: A Laboratory Manual: Volume 2, 2nd ed.; Cold Spring Harbor: New York, NY, USA, 1989; ISBN 978-0-87969-309-1. [Google Scholar]
- Świerzko, A.S.; Cedzyński, M. The Influence of the Lectin Pathway of Complement Activation on Infections of the Respiratory System. Front. Immunol. 2020, 11, 585243. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and Visualization of LD and Haplotype Maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, R.; Thiel, S.; Varming, K.; Jersild, C.; Jensenius, J.C. Detection of Structural Gene Mutations and Promoter Polymorphisms in the Mannan-Binding Lectin (MBL) Gene by Polymerase Chain Reaction with Sequence-Specific Primers. J. Immunol. Methods 2000, 241, 33–42. [Google Scholar] [CrossRef]
- Zhang, N.; Zhuang, M.; Ma, A.; Wang, G.; Cheng, P.; Yang, Y.; Wang, X.; Zhang, J.; Chen, X.; Lu, M. Association of Levels of Mannose-Binding Lectin and the MBL2 Gene with Type 2 Diabetes and Diabetic Nephropathy. PLoS ONE 2013, 8, e83059. [Google Scholar] [CrossRef]
- Yarden, J.; Radojkovic, D.; De Boeck, K.; Macek, M., Jr.; Zemkova, D.; Vavrova, V.; Vlietinck, R.; Cassiman, J.-J.; Cuppens, H. Polymorphisms in the Mannose Binding Lectin Gene Affect the Cystic Fibrosis Pulmonary Phenotype. J. Med. Genet. 2004, 41, 629–633. [Google Scholar] [CrossRef][Green Version]
- Laisk, T.; Peters, M.; Saare, M.; Haller-Kikkatalo, K.; Karro, H.; Salumets, A. Association of CCR5, TLR2, TLR4 and MBL Genetic Variations with Genital Tract Infections and Tubal Factor Infertility. J. Reprod. Immunol. 2010, 87, 74–81. [Google Scholar] [CrossRef]
- Tarova, E.T.; Polakova, H.; Kayserova, H.; Celec, P.; Zuzulova, M.; Kadasi, L. Study of the Effect of DNA Polymorphisms in the Mannose-Binding Lectin Gene (MBL2) on Disease Severity in Slovak Cystic Fibrosis Patients. Gen. Physiol. Biophys. 2012, 30, 373–378. [Google Scholar] [CrossRef]
- Sokołowska, A.; Świerzko, A.S.; Gajek, G.; Gołos, A.; Michalski, M.; Nowicki, M.; Szala-Poździej, A.; Wolska-Washer, A.; Brzezińska, O.; Wierzbowska, A.; et al. Associations of Ficolins and Mannose-Binding Lectin with Acute Myeloid Leukaemia in Adults. Sci. Rep. 2020, 10, 10561. [Google Scholar] [CrossRef] [PubMed]
- Swale, A.; Miyajima, F.; Kolamunnage-Dona, R.; Roberts, P.; Little, M.; Beeching, N.J.; Beadsworth, M.B.J.; Liloglou, T.; Pirmohamed, M. Serum Mannose-Binding Lectin Concentration, but Not Genotype, Is Associated With Clostridium Difficile Infection Recurrence: A Prospective Cohort Study. Clin. Infect. Dis. 2014, 59, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.C.; Levy, E.R.; Madden, K.; Agan, A.A.; Sullivan, R.M.; Graham, D.A.; Randolph, A.G. Mannose-Binding Lectin Levels in Critically Ill Children With Severe Infections. Pediatr. Crit. Care Med. 2017, 18, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lin, Y.; Cai, L.; Mao, Q.; Niu, J. Association between Mannose-Binding Lectin Variants, Haplotypes and Risk of Hepatocellular Carcinoma: A Case-Control Study. Sci. Rep. 2016, 6, 32147. [Google Scholar] [CrossRef]
- Puente, M.; Fariñas-Alvarez, C.; Moreto, A.; Sánchez-Velasco, P.; Ocejo-Vinyals, J.G.; Fariñas, M.C.; on behalf of SCT Team. Low Pre-Transplant Levels of Mannose-Binding Lectin Are Associated with Viral Infections and Mortality after Haematopoietic Allogeneic Stem Cell Transplantation. BMC Immunol. 2019, 20, 40. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Sharma, S.; Arora, H.; Kaur, M. Genetic and Phenotypic Screening of Mannose-Binding Lectin in Relation to Risk of Recurrent Vulvovaginal Infections in Women of North India: A Prospective Cohort Study. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Losada López, I.; García Gasalla, M.; González Moreno, J.; Serrano, A.; Domínguez Valdés, F.J.; Milà, J.; Payeras, A. Mannose Binding Lectin Polymorphisms in Systemic Lupus Erythematosus in Spain. Eur. J. Inflamm. 2016, 14, 78–85. [Google Scholar] [CrossRef]
- Pągowska-Klimek, I.; Świerzko, A.S.; Michalski, M.; Moll, M.; Szala-Poździej, A.; Sokołowska, A.; Krajewski, W.R.; Cedzyński, M. Mannose-Binding Lectin (MBL) Insufficiency Protects against the Development of Systemic Inflammatory Response after Pediatric Cardiac Surgery. Immunobiology 2016, 221, 175–181. [Google Scholar] [CrossRef]
- Gu, X.; Ji, Q.; Wang, H.; Jiang, M.; Yang, J.; Fang, M.; Wang, M.; Gao, C. Genetic Variants of Mannose-Binding Lectin 2 Gene Influence Progression and Prognosis of Patients with Hepatitis B Virus Infection in China. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Monsey, L.; Best, L.G.; Zhu, J.; DeCroo, S.; Anderson, M.Z. The Association of Mannose Binding Lectin Genotype and Immune Response to Chlamydia Pneumoniae: The Strong Heart Study. PLoS ONE 2019, 14, e0210640. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Sabooteh, T.; Shahsavar, F.; Anbari, K.; Pouremadi, F. Mannose-Binding Lectin (MBL) Gene Polymorphisms in Susceptibility to Pulmonary Tuberculosis among the Lur Population of Lorestan Province of Iran. Genom. Data 2017, 12, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Zhu, L.; Shi, M.; Yu, S.; Jin, Y.; Wang, Z.; Ma, J.; Yang, M.; Zhang, X.; Pan, X.; et al. A Rare Genetic Defect of MBL2 Increased the Risk for Progression of IgA Nephropathy. Front. Immunol. 2019, 10, 537. [Google Scholar] [CrossRef]
- Giang, N.T.; van Tong, H.; Quyet, D.; Hoan, N.X.; Nghia, T.H.; Nam, N.M.; Hung, H.V.; Anh, D.T.; Van Mao, C.; Son, H.A.; et al. Complement Protein Levels and MBL2 Polymorphisms Are Associated with Dengue and Disease Severity. Sci. Rep. 2020, 10, 14923. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, L.; Vuononvirta, J.; Mertsola, J.; Waris, M.; He, Q.; Peltola, V. Polymorphisms of Mannose-Binding Lectin and Toll-like Receptors 2, 3, 4, 7 and 8 and the Risk of Respiratory Infections and Acute Otitis Media in Children. Pediatr. Infect. Dis. J. 2017, 36, e114–e122. [Google Scholar] [CrossRef] [PubMed]
- Bujalkova, M.; Zavodna, K.; Krivulcik, T.; Ilencikova, D.; Wolf, B.; Kovac, M.; Karner-Hanusch, J.; Heinimann, K.; Marra, G.; Jiricny, J.; et al. Multiplex SNaPshot Genotyping for Detecting Loss of Heterozygosity in the Mismatch-Repair Genes MLH1 and MSH2 in Microsatellite-Unstable Tumors. Clin. Chem. 2008, 54, 1844–1854. [Google Scholar] [CrossRef]
- Mehta, B.; Daniel, R.; Phillips, C.; McNevin, D. Forensically Relevant SNaPshot® Assays for Human DNA SNP Analysis: A Review. Int. J. Leg. Med. 2017, 131, 21–37. [Google Scholar] [CrossRef]
- Garred, P.; Strøm, J.J.; Quist, L.; Taaning, E.; Madsen, H.O. Association of Mannose-Binding Lectin Polymorphisms with Sepsis and Fatal Outcome, in Patients with Systemic Inflammatory Response Syndrome. J. Infect. Dis. 2003, 188, 1394–1403. [Google Scholar] [CrossRef]
- Skalníková, H.; Freiberger, T.; Chumchalová, J.; Grombiříková, H.; Šedivá, A. Cost-Effective Genotyping of Human MBL2 Gene Mutations Using Multiplex PCR. J. Immunol. Methods 2004, 295, 139–147. [Google Scholar] [CrossRef]
- de Araujo, F.J.; Mesquita, T.G.; da Silva, L.D.O.; de Almeida, S.A.; de S Vital, W.; Chrusciak-Talhari, A.; de O Guerra, J.A.; Talhari, S.; Ramasawmy, R. Functional Variations in MBL2 Gene Are Associated with Cutaneous Leishmaniasis in the Amazonas State of Brazil. Genes Immun. 2015, 16, 284–288. [Google Scholar] [CrossRef]
- Speletas, M.; Gounaris, A.; Sevdali, E.; Kompoti, M.; Konstantinidi, K.; Sokou, R.; Tsitsami, E.; Germenis, A.E. MBL2 Genotypes and Their Associations with MBL Levels and NICU Morbidity in a Cohort of Greek Neonates. J. Immunol. Res. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Munthe-Fog, L.; Madsen, H.O.; Garred, P. Genotyping of FCN and MBL2 Polymorphisms Using Pyrosequencing. In The Complement System; Gadjeva, M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; Volume 1100, pp. 123–130. ISBN 978-1-62703-723-5. [Google Scholar]
- Konopac, M.; Dusatkova, P.; Cinek, O. SNPman: A Program for Genotype Calling Using Run Data from TaqMan Allelic Discrimination. Bioinformatics 2011, 27, 2306–2308. [Google Scholar] [CrossRef]
- Ito, T.; Inoue, E.; Kamatani, N. Association Test Algorithm Between a Qualitative Phenotype and a Haplotype or Haplotype Set Using Simultaneous Estimation of Haplotype Frequencies, Diplotype Configurations and Diplotype-Based Penetrances. Genetics 2004, 168, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Wang, K.; Luo, X. Use of Diplotypes—Matched Haplotype Pairs from Homologous Chromosomes—in Gene-Disease Association Studies. Shanghai Arch. Psychiatry 2014, 26, 165–170. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Osumi, T.; Uchiyama, M.; Shirai, R.; Moriyama, T.; Nishii, R.; Yamada, Y.; Kudo, K.; Sekiguchi, M.; Arakawa, Y.; et al. Diplotype Analysis of NUDT15 Variants and 6-Mercaptopurine Sensitivity in Pediatric Lymphoid Neoplasms. Leukemia 2018, 32, 2710–2714. [Google Scholar] [CrossRef]
- Al Bkhetan, Z.; Zobel, J.; Kowalczyk, A.; Verspoor, K.; Goudey, B. Exploring Effective Approaches for Haplotype Block Phasing. BMC Bioinform. 2019, 20, 540. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Haplotype Phasing: Existing Methods and New Developments. Nat. Rev. Genet. 2011, 12, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin Suite Ver 3.5: A New Series of Programs to Perform Population Genetics Analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Kim, S.; Park, K.; Shin, C.; Cho, N.H.; Ko, J.-J.; Koh, I.; Kwack, K. Diplotyper: Diplotype-Based Association Analysis. BMC Med. Genom. 2013, 6, S5. [Google Scholar] [CrossRef] [PubMed]
- Kavrikova, D.; Borilova Linhartova, P.; Lucanova, S.; Poskerova, H.; Fassmann, A.; Izakovicova Holla, L. Chemokine Receptor 2 (CXCR2) Gene Variants and Their Association with Periodontal Bacteria in Patients with Chronic Periodontitis. Mediat. Inflamm. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n = 328 Total | n = 192 Female | n = 136 Male |
|---|---|---|---|
| Age (mean ± SD, median, IQR) in years | 34.0 ± 13.5, 30.0, 23.0–43.0 | 34.0 ± 14.3, 27.0, 22.0–44.5 | 34.1 ± 12.2, 31.0, 25.0–41.0 |
| Rs Number | Nucleotide Substitution 1 (Location, Amino Acid Substitution/SNP Position 2) | Allele | Primer Sequence (5’→ 3’) | DNA Strand | Concentration of the Primer in the SBE Primer Mix (μM) |
|---|---|---|---|---|---|
| rs1800450 | 161G>A (exon 1, Gly54Asp) | A/B | CCAGGCAAAGATGGGYGTGATG 3 | forward | 0.1 |
| rs1800451 | 170G>A (exon 1, Gly57Glu) | A/C | TTTTACGTACCTGGTTCCCCCTTTTCT | reverse | 0.18 |
| rs7096206 | −290C>G (promoter, -221 2) | X/Y | TTTTTTTTGGTCCCATTTGTTCTCACTGCCAC | forward | 0.06 |
| rs5030737 | 154C>T (exon 1, Arg52Cys) | A/D | TTTTTTTTTTTTCCCTTTTCTYCCTTGGTGYCATCAC 3 | reverse | 0.08 |
| rs11003125 | −619C>G (promoter, -550 2) | H/L | TTTTTTTTTTTTTTTTGGAGTTTGCTTCCCCTTGGTGTTTTA | reverse | 0.08 |
| rs7095891 | −66C>T (5’ UTR of exon 1, +4 2) | P/Q | TTTTTTTTCAGGGAAGGTTAATCTCAGTTAATGAACACATATTTACC | reverse | 0.06 |
| rs Number | Allele | MAF (%) | |||||
|---|---|---|---|---|---|---|---|
| n = 328 Czech | n = 1006 EUR | n = 1322 AFR | n = 1008 EAS | n = 978 SAS | n = 694 AMR | ||
| rs11003125 | H | 38.3 | 38.6 | 8.7 | 44.6 | 31.0 | 40.0 |
| rs7096206 | X | 21.4 | 22.1 | 15.4 | 18.6 | 28.0 | 13.0 |
| rs7095891 | Q | 23.6 | 19.8 | 54.0 | 13.8 | 25.0 | 17.0 |
| rs5030737 | D | 8.5 | 6.0 | 0.2 | 0.1 | 5.0 | 3.0 |
| rs1800450 | B | 13.5 | 14.1 | 1.4 | 14.8 | 15.0 | 22.0 |
| rs1800451 | C | 1.8 | 1.2 | 25.9 | 0.0 | 4.0 | 2.0 |
| MBL2 Haplotype 1 | Combination of Alleles (Secretor Haplotype) | MBL Phenotype 2 | Frequency in Czech Population (%) | Frequency in Danish Population 3 (%) |
|---|---|---|---|---|
| G G C C G G | HYPA | High | 29.8 | 28.5 |
| C G T C G G | LYQA | High | 21.7 | 23.5 |
| C C C C G G | LXPA | Low | 21.4 | 19.5 |
| C G C C A G | LYPB | Undetectable | 13.3 | 13.5 |
| G G C T G G | HYPD | Undetectable | 8.5 | 8.5 |
| C G C C G G | LYPA | Intermediate | 3.3 | 4.5 |
| C G T C G A | LYQC | Undetectable | 1.8 | 2.0 |
| C G T C A G | LYQB | Undetectable | 0.2 | - |
| MBL2 Haplogenotype 1 | Combination of Secretor Haplotypes 2 | Frequency (%) |
|---|---|---|
| GC GG CT CC GG GG | HYPA/LYQA | 16.2 |
| GC GC CC CC GG GG | HYPA/LXPA | 11.9 |
| CC GC CT CC GG GG | LYQA/LXPA | 10.1 |
| GG GG CC CC GG GG | HYPA/HYPA | 7.9 |
| GC GG CC CC GA GG | HYPA/LYPB | 7.3 |
| CC GG CT CC GA GG | LYQA/LYPB | 6.1 |
| CC CC CC CC GG GG | LXPA/LXPA | 5.5 |
| GG GG CC CT GG GG | HYPA/HYPD | 5.2 |
| CC GC CC CC GA GG | LXPA/LYPB | 4.0 |
| CC GG TT CC GG GG | LYQA/LYQA | 3.7 |
| GC GG CC CT GA GG | LYPB/HYPD | 3.0 |
| GC GC CC CT GG GG | LXPA/HYPD | 3.0 |
| GC GG CT CT GG GG | LYQA/HYPD | 2.4 |
| GC GG CC CC GG GG | HYPA/LYPA | 2.4 |
| CC GG CC CC AA GG | LYPB/LYPB | 2.1 |
| CC GC CT CC GG GA | LXPA/LYQC | 1.8 |
| CC GG CC CC GA GG | LYPB/LYPA | 1.2 |
| CC GG CT CC GG GG | LYQA/LYPA | 1.2 |
| GC GG CC CT GG GG | HYPD/LYPA | 0.9 |
| CC GC CC CC GG GG | LXPA/LYPA | 0.9 |
| GG GG CC TT GG GG | HYPD/HYPD | 0.9 |
| GC GG CT CC GG GA | HYPA/LYQC | 0.6 |
| CC GG CT CC GA GA | LYPB/LYQC | 0.6 |
| CC GG TT CC GG GA | LYQA/LYQC | 0.3 |
| GC GG CT CT GG GA | HYPD/LYQC | 0.3 |
| GC GG CT CC GA GG | HYPA/LYQB | 0.3 |
| Allele -Specific PCR (AS-PCR) | ARMS 3/Double ARMS 3 (+ Multiplex Allele -Specific PCR) | PCR and Restriction-Fragment Length Polymorphism (PCR-RFLP) | Commercial TaqMan Assay 5 | High-Resolution Melt Analysis (HRMA) | Commercial INNO-LiPA MBL2 kit (Reverse PCR-SSOP) | Pyro- Sequencing | Sanger Sequencing | MBL2 SNaPshot Assay | |
|---|---|---|---|---|---|---|---|---|---|
| principle of allele discrimination/detection | PCR with a primer specific for one allele | PCR with primers specific for both alleles | allele-specific enzymatic cleavage of PCR amplicon | allele-specific hybridization of fluorescently labelled probe | temperature-dependent allele-specific hybridization of fluorescently labelled probe | hybridization of biotinylated PCR product with membrane immobilized sequence-specific oligonucleotide probes | chemiluminiscence-based detection of nucleotides during sequencing-by-synthesis reaction | detection of the sequence of an oligonucleotide amplified in PCR with fluorescently labelled dideoxyribonucleotides | allele-specific SBE by a single fluorescently labelled dideoxyribonucleotide (minisequencing) |
| post-PCR analysis | yes | yes | yes | no | no, when real-time PCR thermocycler is used | yes | no | yes | yes |
| analysis time | 2 h 2 | 2–3 h 2 | 2 h + 1–3 h 4 | 1–2 h 6 | 1–1.5 h + 2–8 min. 8 | 3–4 h | 2–3 h | 6–7 h | 5–6 h |
| number of work steps | 2 (PCR, gel analysis) | 2 (PCR, gel analysis) | 4 (PCR, gel analysis, RFLP, gel analysis) | 1 (real-time PCR) | 1 (when real-time PCR thermocycler is used for PCR and subsequent melting temperature analysis) | 9 (PCR, gel analysis, denaturation, hybridization, 2 washing steps, 3-step color development) | 4 (PCR, gel analysis, purification, pyrosequencing) | 5 (PCR, enzymatic cleaning, sequencing reaction, purification, analysis on sequencer) | 5 (PCR, enzymatic cleaning, SBE reaction, enzymatic cleaning, analysis on sequencer) |
| automatic analysis | no | no | no | yes | yes | no | yes | yes | yes |
| number of analyses for complete MBL2 haplogenotype 1 | 12 | 6 | 6 | 6 | 5 | 1 | 4 9 | 2 | 1 |
| number of oligonucleotide primers + labelled primers/probes for complete MBL2 haplogenotype 1 | 24 primers | 15 primers | 6 primers | 6 TaqMan assays (12 primers + 12 TaqMan probes) | 10 primers + 5 TaqMan probes | 4 primers | 8 primers + 4 biotinylated primers | 2 primers | 8 primers |
| estimated cost of analysis of whole haplogenotype 1 | 1 USD | 1 USD | 2 USD | 2 USD | 1 USD | product was discontinued | 2 USD | 5 USD | 1.50 USD |
| input amount of template DNA | 20–200 ng | 20–200 ng | 50–500 ng | 1–20 ng | 10–20 ng | 200–500 ng | 10–100 ng | 10–250 ng | 10–100 ng |
| assay robustness | low | low | low–medium | medium–high | high | low–medium | medium | medium–high | medium–high |
| special equipment requirement | - | - | - | real-time PCR thermocycler | real-time PCR thermocycler or fluorescence scanning/detection system | water bath with shaking platform, aspiration apparatus | vacuum prep workstation, pyrosequencing machine | automated DNA sequencer | automated DNA sequencer |
| SNP genotyping throughput | low | low | low | high | high | medium | high | high | high |
| software for automatic allele calling | no | no | no | yes (SDS software, SNPman program) 7 | yes real-time PCR instruments with HRMA compatible software with genotype auto-calling function | no | no | yes (Mutation Surveyor, GeneMarker, Minor Variant Finder Software, SeqScape™ Software, Variant Reporter™ Software) 10 | yes (GeneMapper, GeneMarker) 11 |
| Ref. | [41] | [30,42] | [43,44] | [27] | [28] | [29] | [45] | [37] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrazkova, J.; Sistek, P.; Lochman, J.; Izakovicova Holla, L.; Danek, Z.; Borilova Linhartova, P. A SNaPshot Assay for Determination of the Mannose-Binding Lectin Gene Variants and an Algorithm for Calculation of Haplogenotype Combinations. Diagnostics 2021, 11, 301. https://doi.org/10.3390/diagnostics11020301
Mrazkova J, Sistek P, Lochman J, Izakovicova Holla L, Danek Z, Borilova Linhartova P. A SNaPshot Assay for Determination of the Mannose-Binding Lectin Gene Variants and an Algorithm for Calculation of Haplogenotype Combinations. Diagnostics. 2021; 11(2):301. https://doi.org/10.3390/diagnostics11020301
Chicago/Turabian StyleMrazkova, Jana, Petr Sistek, Jan Lochman, Lydie Izakovicova Holla, Zdenek Danek, and Petra Borilova Linhartova. 2021. "A SNaPshot Assay for Determination of the Mannose-Binding Lectin Gene Variants and an Algorithm for Calculation of Haplogenotype Combinations" Diagnostics 11, no. 2: 301. https://doi.org/10.3390/diagnostics11020301
APA StyleMrazkova, J., Sistek, P., Lochman, J., Izakovicova Holla, L., Danek, Z., & Borilova Linhartova, P. (2021). A SNaPshot Assay for Determination of the Mannose-Binding Lectin Gene Variants and an Algorithm for Calculation of Haplogenotype Combinations. Diagnostics, 11(2), 301. https://doi.org/10.3390/diagnostics11020301






