Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters
Abstract
1. Introduction
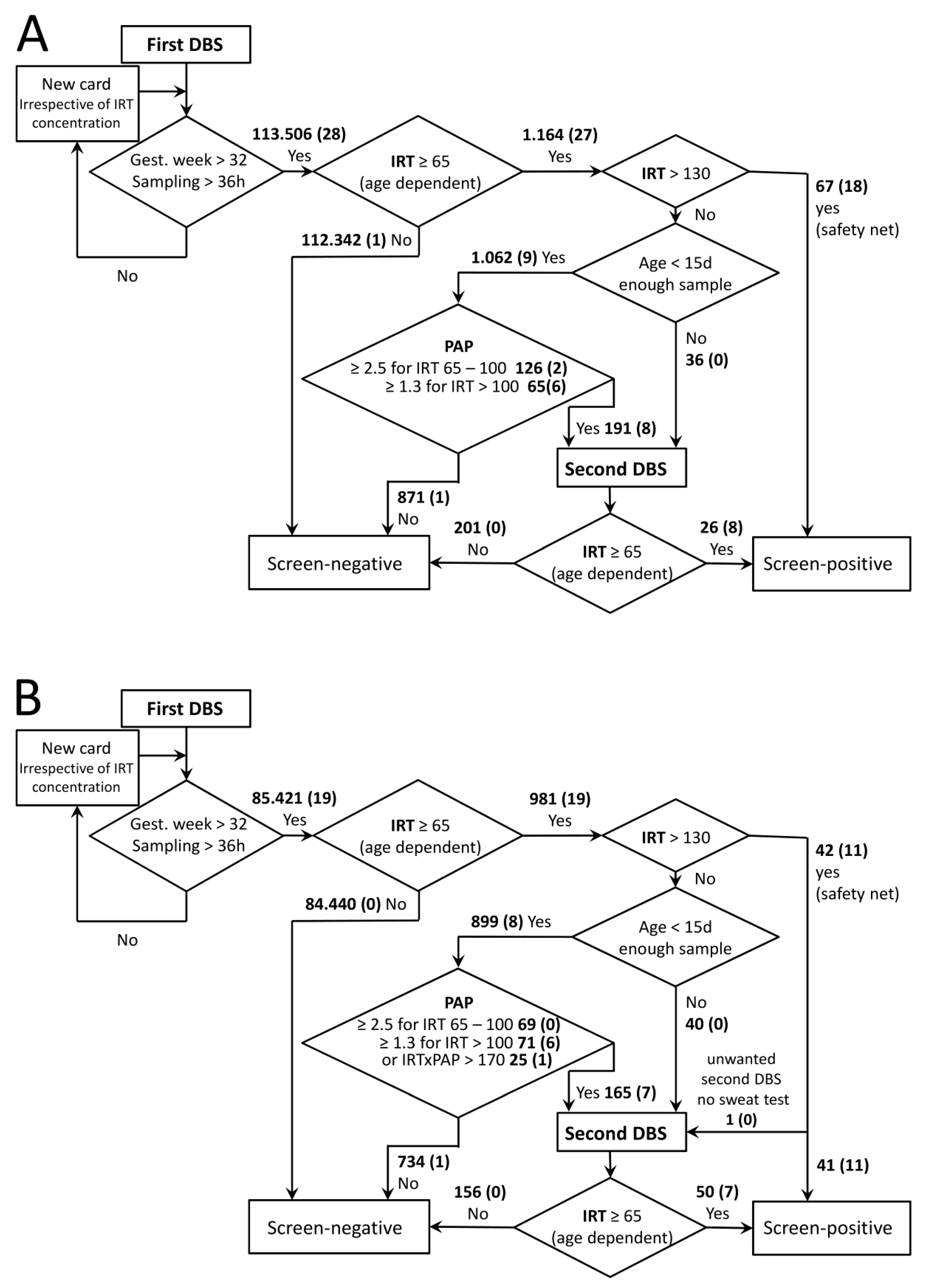
2. Materials and Methods
2.1. Patients
2.2. DBS Tests
2.3. CF Diagnosis
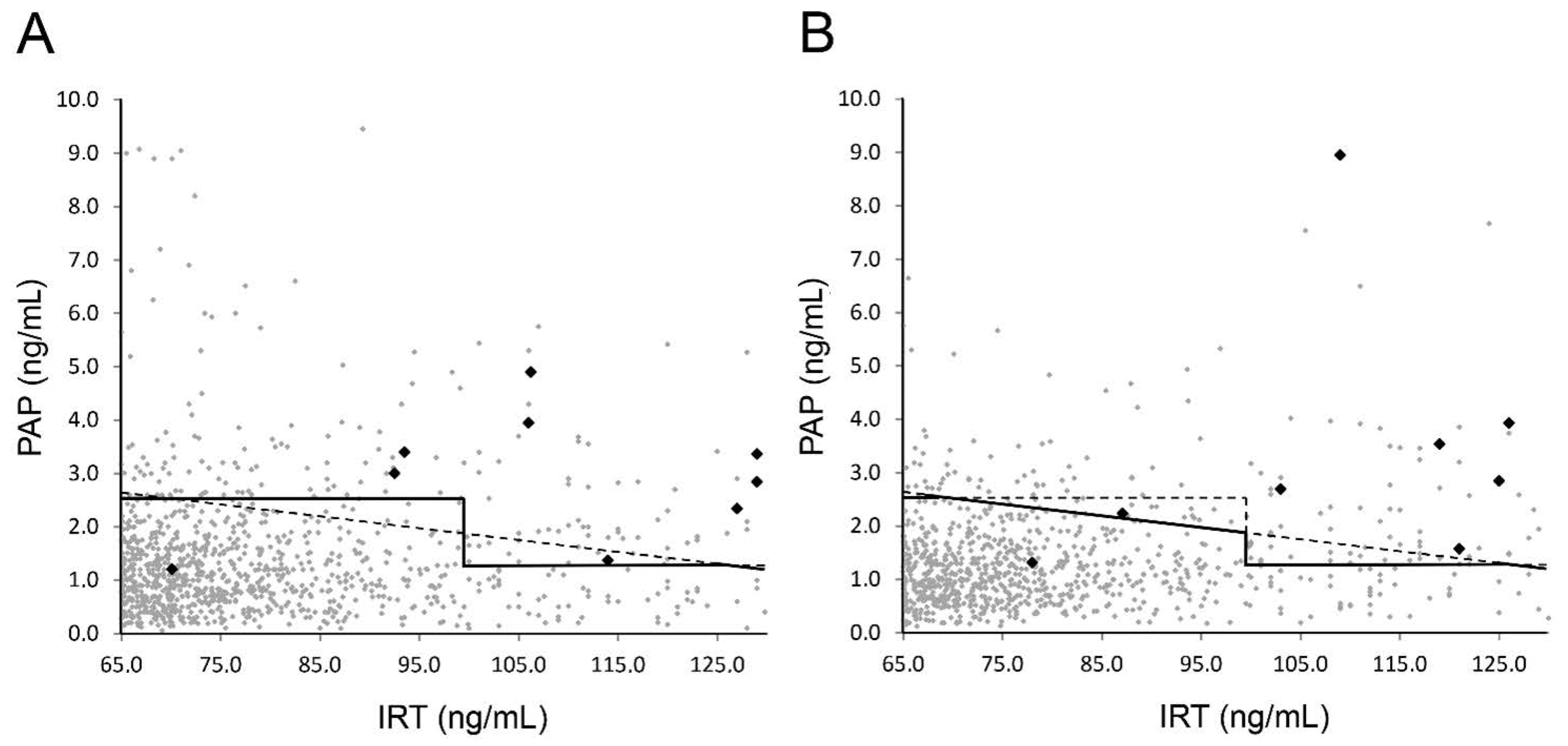
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, S.D.; White, R.; Tobin, P. Keep them breathing: Cystic fibrosis pathophysiology, diagnosis, and treatment. J. Am. Acad. PAs 2017, 30, 23–27. [Google Scholar] [CrossRef]
- Grosse, S.D.; Rosenfeld, M.; Devine, O.J.; Lai, H.J.; Farrell, P.M. Potential impact of newborn screening for cystic fibrosis on child survival: A systematic review and analysis. J. Pediatr. 2006, 149, 362–366. [Google Scholar] [CrossRef]
- Dijk, F.N.; McKay, K.; Barzi, F.; Gaskin, K.J.; Fitzgerald, D.A. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch. Dis. Child. 2011, 96, 1118–1123. [Google Scholar] [CrossRef]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef]
- Crossley, J.R.; Elliott, R.B.; Smith, P.A. Dried-blood spot screening for cystic fibrosis in the newborn. Lancet 1979, 1, 472–474. [Google Scholar] [CrossRef]
- Schmidt, M.; Werbrouck, A.; Verhaeghe, N.; De Wachter, E.; Simoens, S.; Annemans, L.; Putman, K. Strategies for newborn screening for cystic fibrosis: A systematic review of health economic evaluations. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2018. [Google Scholar] [CrossRef]
- De Boeck, K.; Vermeulen, F.; Dupont, L. The diagnosis of cystic fibrosis. Presse Med. (Paris France 1983) 2017, 46, e97–e108. [Google Scholar] [CrossRef]
- Pollak, A.; Kasper, D.C. Austrian Newborn Screening Program: A perspective of five decades. J. Perinat. Med. 2014, 42, 151–158. [Google Scholar] [CrossRef]
- Frischer, T.; Eber, E.; Ellemunter, H.; Zacharasiewicz, A.; Kaluza, I.; Riedler, J.; Renner, S. Cystic fibrosis in Austria. Wien. Klin. Wochenschr. 2017, 129, 527–532. [Google Scholar] [CrossRef]
- Barben, J.; Castellani, C.; Dankert-Roelse, J.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.; Munck, A.; Sands, D.; Sommerburg, O.; et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J. Cyst. Fibros. 2017, 16, 207–213. [Google Scholar] [CrossRef]
- Barthellemy, S.; Maurin, N.; Roussey, M.; Ferec, C.; Murolo, S.; Berthezene, P.; Iovanna, J.L.; Dagorn, J.C.; Sarles, J. Evaluation of 47,213 infants in neonatal screening for cystic fibrosis, using pancreatitis-associated protein and immunoreactive trypsinogen assays. Arch. Pediatrie Organe Off. La Soc. Fr. Pediatrie 2001, 8, 275–281. [Google Scholar] [CrossRef]
- Baker, M.W.; Groose, M.; Hoffman, G.; Rock, M.; Levy, H.; Farrell, P.M. Optimal DNA tier for the IRT/DNA algorithm determined by CFTR mutation results over 14 years of newborn screening. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2011, 10, 278–281. [Google Scholar] [CrossRef][Green Version]
- Cordovado, S.K.; Hendrix, M.; Greene, C.N.; Mochal, S.; Earley, M.C.; Farrell, P.M.; Kharrazi, M.; Hannon, W.H.; Mueller, P.W. CFTR mutation analysis and haplotype associations in CF patients. Mol. Genet. Metab. 2012, 105, 249–254. [Google Scholar] [CrossRef]
- Seror, V.; Cao, C.; Roussey, M.; Giorgi, R. PAP assays in newborn screening for cystic fibrosis: A population-based cost-effectiveness study. J. Med. Screen 2016, 23, 62–69. [Google Scholar] [CrossRef]
- Raimond, V.; Sambuc, C.; Pibouleau, L. Ethics Evaluation Revealing Decision-Maker Motives: A Case of Neonatal Screening. Int. J. Technol. Assess. Health Care 2018, 34, 189–195. [Google Scholar] [CrossRef]
- Sommerburg, O.; Krulisova, V.; Hammermann, J.; Lindner, M.; Stahl, M.; Muckenthaler, M.; Kohlmueller, D.; Happich, M.; Kulozik, A.E.; Votava, F.; et al. Comparison of different IRT-PAP protocols to screen newborns for cystic fibrosis in three central European populations. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2014, 13, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Krulisova, V.; Balascakova, M.; Skalicka, V.; Piskackova, T.; Holubova, A.; Paderova, J.; Krenkova, P.; Dvorakova, L.; Zemkova, D.; Kracmar, P.; et al. Prospective and parallel assessments of cystic fibrosis newborn screening protocols in the Czech Republic: IRT/DNA/IRT versus IRT/PAP and IRT/PAP/DNA. Eur. J. Pediatr. 2012, 171, 1223–1229. [Google Scholar] [CrossRef]
- Sarles, J.; Giorgi, R.; Berthezene, P.; Munck, A.; Cheillan, D.; Dagorn, J.C.; Roussey, M. Neonatal screening for cystic fibrosis: Comparing the performances of IRT/DNA and IRT/PAP. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2014, 13, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Hammermann, J.; Lindner, M.; Stahl, M.; Muckenthaler, M.; Kohlmueller, D.; Happich, M.; Kulozik, A.E.; Stopsack, M.; Gahr, M.; et al. Five years of experience with biochemical cystic fibrosis newborn screening based on IRT/PAP in Germany. Pediatric Pulmonol. 2015, 50, 655–664. [Google Scholar] [CrossRef]
- Vernooij-van Langen, A.M.; Loeber, J.G.; Elvers, B.; Triepels, R.H.; Gille, J.J.; Van der Ploeg, C.P.; Reijntjens, S.; Dompeling, E.; Dankert-Roelse, J.E. Novel strategies in newborn screening for cystic fibrosis: A prospective controlled study. Thorax 2012, 67, 289–295. [Google Scholar] [CrossRef]
- Weidler, S.; Stopsack, K.H.; Hammermann, J.; Sommerburg, O.; Mall, M.A.; Hoffmann, G.F.; Kohlmuller, D.; Okun, J.G.; Macek, M., Jr.; Votava, F.; et al. A product of immunoreactive trypsinogen and pancreatitis-associated protein as second-tier strategy in cystic fibrosis newborn screening. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2016, 15, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Vernooij-van Langen, A.M.; Loeber, J.G.; Elvers, B.; Triepels, R.H.; Roefs, J.; Gille, J.J.; Reijntjens, S.; Dompeling, E.; Dankert-Roelse, J.E. The influence of sex, gestational age, birth weight, blood transfusion, and timing of the heel prick on the pancreatitis-associated protein concentration in newborn screening for cystic fibrosis. J. Inherit. Metab. Dis. 2013, 36, 147–154. [Google Scholar] [CrossRef]
- Adam, B.W.; Hall, E.M.; Sternberg, M.; Lim, T.H.; Flores, S.R.; O’Brien, S.; Simms, D.; Li, L.X.; De Jesus, V.R.; Hannon, W.H. The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin. Biochem. 2011, 44, 1445–1450. [Google Scholar] [CrossRef]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2018, 17, 153–178. [Google Scholar] [CrossRef]
- Castellani, C.; Southern, K.W.; Brownlee, K.; Dankert Roelse, J.; Duff, A.; Farrell, M.; Mehta, A.; Munck, A.; Pollitt, R.; Sermet-Gaudelus, I.; et al. European best practice guidelines for cystic fibrosis neonatal screening. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2009, 8, 153–173. [Google Scholar] [CrossRef]
- Munck, A.; Mayell, S.J.; Winters, V.; Shawcross, A.; Derichs, N.; Parad, R.; Barben, J.; Southern, K.W. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J. Cyst. Fibros. 2015, 14, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Doull, I. Devil in the detail of newborn screening for cystic fibrosis. Arch. Dis. Child. 2018. [Google Scholar] [CrossRef]
- Castellani, C.; Picci, L.; Tridello, G.; Casati, E.; Tamanini, A.; Bartoloni, L.; Scarpa, M.; Assael, B.M. Cystic fibrosis carrier screening effects on birth prevalence and newborn screening. Genet. Med. 2016, 18, 145–151. [Google Scholar] [CrossRef]
- Munck, A. Inconclusive Diagnosis after Newborn Screening for Cystic Fibrosis. Int. J. Neonatal Screen. 2020, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Bergougnoux, A.; Lopez, M.; Girodon, E. The Role of Extended CFTR Gene Sequencing in Newborn Screening for Cystic Fibrosis. Int. J. Neonatal Screen. 2020, 6, 23. [Google Scholar] [CrossRef]
- Esquerda, M.; Palau, F.; Lorenzo, D.; Cambra, F.J.; Bofarull, M.; Cusi, V.; Interdisciplinar en Bioetica, G. Ethical questions concerning newborn genetic screening. Clin. Genet. 2021, 99, 93–98. [Google Scholar] [CrossRef]
- Skov, M.; Bækvad-Hansen, M.; Hougaard, D.M.; Skogstrand, K.; Lund, A.M.; Pressler, T.; Olesen, H.V.; Duno, M. Cystic fibrosis newborn screening in Denmark: Experience from the first 2 years. Pediatric Pulmonol. 2020, 55, 549–555. [Google Scholar] [CrossRef]
- Dankert-Roelse, J.E.; Bouva, M.J.; Jakobs, B.S.; Janssens, H.M.; de Winter-de Groot, K.M.; Schönbeck, Y.; Gille, J.J.P.; Gulmans, V.A.M.; Verschoof-Puite, R.K.; Schielen, P.C.J.I.; et al. Newborn blood spot screening for cystic fibrosis with a four-step screening strategy in the Netherlands. J. Cyst. Fibros. 2019, 18, 54–63. [Google Scholar] [CrossRef]
| ID | IRT 1st DBS (ng/mL) | MucoPAP (ng/mL) | IRT 2nd DBS (ng/mL) | Mutation (Common Name) |
|---|---|---|---|---|
| 1 | 87 | 2.2 | 73 | DF508, homozygous |
| 2 | 93 | 3.0 | 98 | DF508/M1101 |
| 3 | 94 | 3.4 | 68 | 182delT/3849 + 10kbC->T |
| 4 | 103 | 2.7 | n.a. | DF508/R117H/7T9T |
| 5 | 106 | 4.0 | 222 | DF508, homozygous |
| 6 | 106 | 4.9 | 246 | DF508, homozygous |
| 7 | 109 | 9.0 | 73 | DF508, homozygous |
| 8 | 114 | 1.3 | n.a. | DF508, homozygous |
| 9 | 119 | 3.5 | 250 | DF508, homozygous |
| 10 | 121 | 1.6 | 124 | N1303K/R347P |
| 11 | 125 | 2.8 | 113 | DF 508/G542X |
| 12 | 126 | 3.9 | 208 | DF508/R117H/7T9T |
| 13 | 127 | 2.3 | 80 | S466X/R1070Q |
| 14 | 129 | 2.8 | 181 | DF508/G551D |
| 15 | 136 | DF508, homozygous | ||
| 16 | 140 | I807M, homozygous | ||
| 17 | 140 | DF 508/182delT | ||
| 18 | 144 | R553X/R117H/7T | ||
| 19 | 144 | DF508, homozygous | ||
| 20 | 145 | DF508, homozygous | ||
| 21 | 152 | DF508/R117H/7T9T | ||
| 22 | 159 | N1303K/Q39X | ||
| 23 | 161 | DF508, homozygous | ||
| 24 | 165 | S549N/CFTR50kbdel | ||
| 25 | 172 | DF508, homozygous | ||
| 26 | 187 | DF508, homozygous | ||
| 27 | 191 | DF508, homozygous | ||
| 28 | 191 | DF508/I507del | ||
| 29 | 197 | DF508l/R1162X | ||
| 30 | 198 | DF508, homozygous | ||
| 31 | 200 | DF508, homozygous | ||
| 32 | 212 | DF508/G542X | ||
| 33 | 213 | DF508/MET 82 Val | ||
| 34 | 219 | DF508, homozygous | ||
| 35 | 226 | DF508, homozygous | ||
| 36 | 229 | DF508, homozygous | ||
| 37 | 234 | DF508, homozygous | ||
| 38 | 237 | DF508, homozygous | ||
| 39 | 246 | DF508/R1162X | ||
| 40 | 265 | DF508/G542X | ||
| 41 | 269 | DF508, homozygous | ||
| 42 | 307 | DF508/G551D | ||
| 43 | 330 | DF508, homozygous | ||
| 44 | 485 | DF508, homozygous |
| Algorithm | Second Cards | Safety Net | Screen- Positive | PPV | Sensitivity |
|---|---|---|---|---|---|
| Period 1 (IRT-PAP) n = 113.506 | 0.20% (227) 1 | 0.06% (67) 1 | 0.08% (93) | 29.2% 2 (26/89) | 92.8% (26/28) |
| Period 2 (IRT-PAP OR IRT×PAP) n = 85.421 | 0.24% 206) | 0.05% (41) | 0.11% (91) | 22.2% 3 (18/81) | 94.7% (18/19) |
| IRT-PAP only (hypothetical, calculated for period 2) n = 85.421 | 0.21% (181) | 0.05% (41) | 0.10% (88) | 21.8% (17/78) | 89.47% (17/19) |
| IRT×PAP only (hypothetical, calculated for period 2) n = 85.421 | 0.22% (191) | 0.05% (41) | 0.11% (90) | 22.5% (18/80) | 94.7% (18/19) |
| Previous Screening (IRT-IRT, 2 yrs) n = 172,322 | 0.88% (1519) | - | 0.17% (285) | 14% (36/285) | 94.7% (36/38) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeyda, M.; Schanzer, A.; Basek, P.; Bauer, V.; Eber, E.; Ellemunter, H.; Kallinger, M.; Riedler, J.; Thir, C.; Wadlegger, F.; et al. Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters. Diagnostics 2021, 11, 299. https://doi.org/10.3390/diagnostics11020299
Zeyda M, Schanzer A, Basek P, Bauer V, Eber E, Ellemunter H, Kallinger M, Riedler J, Thir C, Wadlegger F, et al. Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters. Diagnostics. 2021; 11(2):299. https://doi.org/10.3390/diagnostics11020299
Chicago/Turabian StyleZeyda, Maximilian, Andrea Schanzer, Pavel Basek, Vera Bauer, Ernst Eber, Helmut Ellemunter, Margit Kallinger, Josef Riedler, Christina Thir, Franz Wadlegger, and et al. 2021. "Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters" Diagnostics 11, no. 2: 299. https://doi.org/10.3390/diagnostics11020299
APA StyleZeyda, M., Schanzer, A., Basek, P., Bauer, V., Eber, E., Ellemunter, H., Kallinger, M., Riedler, J., Thir, C., Wadlegger, F., Zacharasiewicz, A., & Renner, S. (2021). Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters. Diagnostics, 11(2), 299. https://doi.org/10.3390/diagnostics11020299






