USH2A-Related Retinitis Pigmentosa: Staging of Disease Severity and Morpho-Functional Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Data Acquisition
2.3. Electroretinogram Assessment
2.4. Spectral Domain-Optical Coherence Tomography Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCVA | Best Corrected Visual Acuity |
| CS | Cumulative Score |
| ETDRS | Early Treatment for Diabetic Retinopathy Study |
| ERG | Electroretinogram |
| EZ | Ellipsoid Zone |
| F | Female |
| FERG | Focal Electroretinogram |
| HET | Heterozygous |
| HOM | Homozygous |
| LE | Left Eye |
| M | Male |
| NSRP | Non-Syndromic RP |
| OCT | Optical Coherence Tomography |
| OR | Outer Retina |
| USH | Usher |
| RE | Right Eye |
| RORA | RPE and OR Atrophy |
| RP | Retinitis Pigmentosa |
| RPE | Retinal Pigment Epithelium |
| SD | Standard Deviation |
| SD-OCT | Spectral domain-OCT |
| SRI | Sub-RPE illumination |
| VF | Visual Field |
References
- Boughman, J.A.; Vernon, M.; Shaver, K.A. Usher syndrome: Definition and estimate of prevalence from two high-risk populations. J. Chronic Dis. 1983, 36, 595–603. [Google Scholar] [CrossRef]
- Grøndahl, J. Estimation of prognosis and prevalence of retinitis pigmentosa and Usher syndrome in Norway. Clin. Genet. 2008, 31, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hope, C.I.; Bundey, S.; Proops, D.; Fielder, A.R. Usher syndrome in the city of Birmingham–prevalence and clinical classification. Br. J. Ophthalmol. 1997, 81, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, T.; Haim, M.; Parving, A.; Hauch, A.-M. The prevalence of Usher syndrome and other retinal dystrophy-hearing impairment associations. Clin. Genet. 1997, 51, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Spandau, U.H.; Rohrschneider, K. Prevalence and geographical distribution of Usher syndrome in Germany. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Fishman, G.A.; Bozbeyoglu, S.; Massof, R.W.; Kimberling, W. Natural course of visual field loss in patients with Type 2 Usher syndrome. Retina 2007, 27, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, A.; Kritchevsky, S.B.; Ciccarelli, M.L.; Tedesco, S.A.; Macaluso, C.; Kimberling, W.J.; Somes, G.W. Kinetics of visual field loss in Usher syndrome Type II. Investig. Ophthalmol. Vis. Sci. 2004, 45, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Fishman, G.A.; Anderson, R.J.; Grover, S.; Derlacki, D.J. Visual acuity and visual field impairment in Usher syndrome. Arch. Ophthalmol. 1998, 116, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo-Soriano, C.; Erkilic, N.; Baux, D.; Mamaeva, D.; Hamel, C.P.; Meunier, I.; Roux, A.-F.; Kalatzis, V. Genome editing in patient iPSCs corrects the most prevalent USH2A mutations and reveals intriguing mutant mRNA expression profiles. Mol. Ther. Methods Clin. Dev. 2019, 17, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Stingl, K.; Kohl, S.; Ries, J.; Linnert, J.; Nagel-Wolfrum, K. Ataluren for the treatment of Usher syndrome 2A caused by nonsense mutations. Int. J. Mol. Sci. 2019, 20, 6274. [Google Scholar] [CrossRef] [PubMed]
- Zein, W.M.; Falsini, B.; Tsilou, E.T.; Turriff, A.E.; Schultz, J.M.; Friedman, T.B.; Brewer, C.C.; Zalewski, C.K.; King, K.A.; Muskett, J.A.; et al. Cone Responses in Usher syndrome Types 1 and 2 by microvolt electroretinography. Investig. Ophthalmol. Vis. Sci. 2014, 56, 107–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galli-Resta, L.; Placidi, G.; Campagna, F.; Ziccardi, L.; Piccardi, M.; Minnella, A.; Abed, E.; Iovine, S.; Maltese, P.; Bertelli, M.; et al. Central retina functional damage in Usher syndrome Type 2: 22 years of focal macular ERG analysis in a patient population from central and southern Italy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Guymer, R.H.; Rosenfeld, P.J.; Curcio, C.A.; Holz, F.G.; Staurenghi, G.; Freund, K.B.; Schmitz-Valckenberg, S.; Sparrow, J.; Spaide, R.F.; Tufail, A.; et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology 2020, 127, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Lemus, M.; Usmani, B.; Campochiaro, P.A.; Sahel, J.A.; Scholl, H.P.N.; Shah, S.M.A. Classification of disease severity in retinitis pigmentosa. Br. J. Ophthalmol. 2019, 103, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Galli-Resta, L.; Piccardi, M.; Ziccardi, L.; Fadda, A.; Minnella, A.; Marangoni, D.; Placidi, G.; Resta, G.; Falsini, B. Early detection of central visual function decline in cone–rod dystrophy by the use of macular focal cone electroretinogram. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6560–6569. [Google Scholar] [CrossRef] [PubMed]
- Galli-Resta, L.; Falsini, B.; Rossi, G.; Piccardi, M.; Ziccardi, L.; Fadda, A.; Minnella, A.; Marangoni, D.; Placidi, G.; Campagna, F.; et al. Bilateral symmetry of visual function loss in cone–rod dystrophies. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3759–3768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iarossi, G.; Falsini, B.; Piccardi, M. Regional cone dysfunction in retinitis pigmentosa evaluated by flicker ERGs: Relationship with perimetric sensitivity losses. Investig. Ophthalmol. Vis. Sci. 2003, 44, 866–874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bennett, L.D.; Wang, Y.-Z.; Klein, M.; Pennesi, M.E.; Jayasundera, T.; Birch, D.G. Structure/psychophysical relationships in X-linked retinoschisis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef] [PubMed]
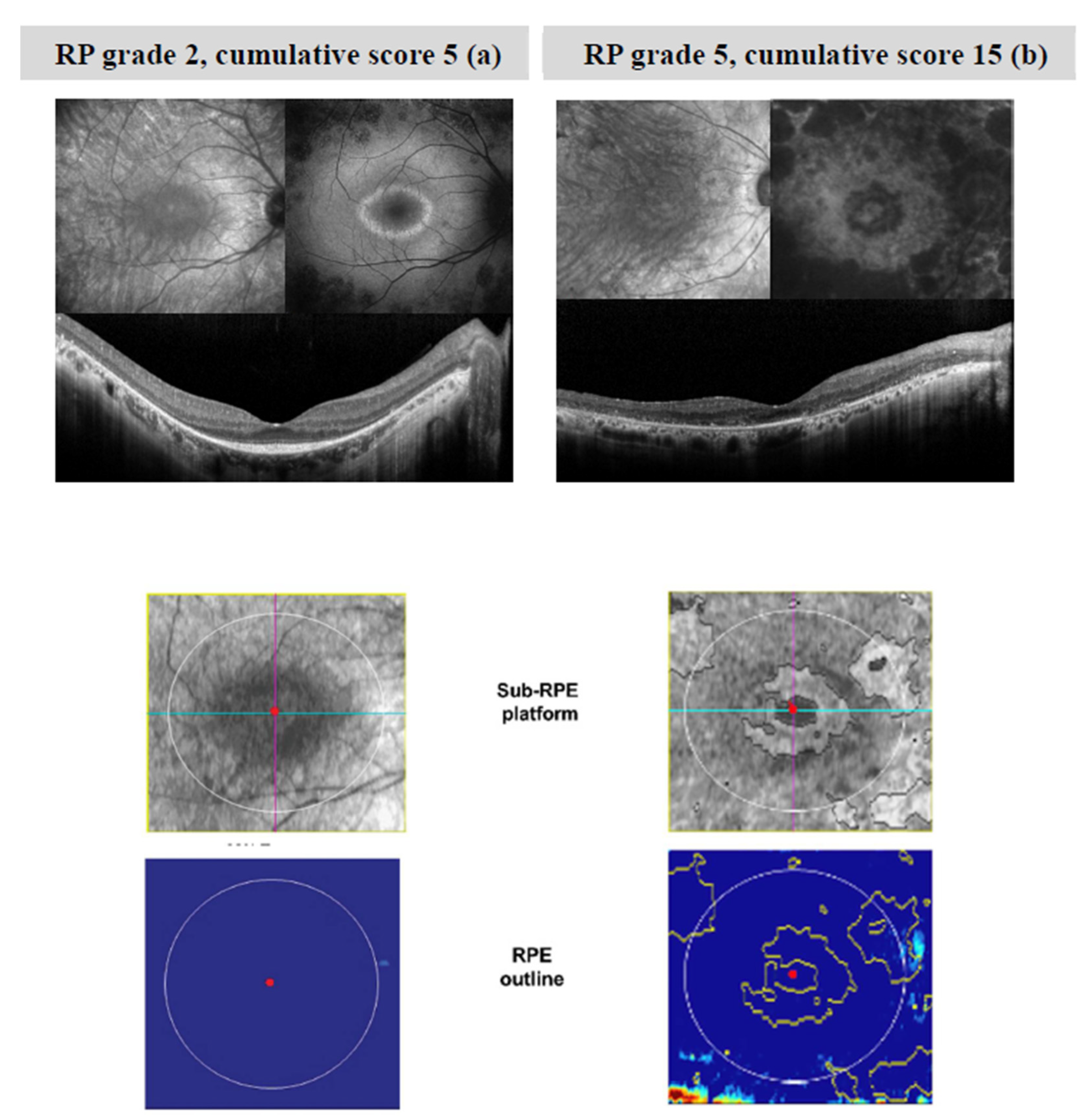



| STATUS | Variant 1 | ACMG | Variant 2 | ACMG | |
|---|---|---|---|---|---|
| 1 | HET | c.1031_1032del; p.(Phe344Cysfs*3) | P | c.6178dup; p.(Gln2060Profs*43) | P |
| 2 | HOM | c.9815C>T; p.(Pro3272Leu) | LP | c.9815C>T; p.(Pro3272Leu) | LP |
| 3 | HET | c.8584C>T; p.(Gln2862*) | P | c.14074G>A; p.(Gly4692Arg) | VUS |
| 4 | HET | c.15199del; p.(Ile5067Serfs*23) | P | c.10712C>T; p.(Thr3571Met) | P |
| 5 | HET | c.990_991del; p.(Asn330Lysfs*8) | P | c.10712C>T; p.(Thr3571Met) | P |
| 6 | HET | c.13130C>A; p.(Ser4377*) | P | c.653T>A; p.(Val218Glu) | LP |
| 7 | HOM | c.14248C>T; p.(Gln4750*) | P | c.14248C>T; p.(Gln4750*) | P |
| 8 | HET | c.10437G>T; p.(Trp3479Cys) | P | c.802G>A; p.(Gly268Arg) | P |
| 9 | HET | c.12067-2A>G; p.(?) | P | duplication in exons 10–14 | VUS |
| 10 | HET | c.8584C>T; p.(Gln2862*) | P | c.13018G>C; p.(Gly4340Arg) | LP |
| 11 | HOM | c.10699del; p.(Leu3567*) | P | c.10699del; p.(Leu3567*) | P |
| 12 | HET | c.2276G>T; p.(Cys759Phe) | P | c.14286C>A; p.(Asn4762Lys) | VUS |
| 13 | HET | c.1055C>T; p.(Thr352Ile) | P | c.10712C>T; p.(Thr3571Met) | P |
| 14 | HET | c.13257_13263del; p.(Phe4419Leufs1*) | P | c.(2809+1_2810-1)_(2993+1_2994-1)del; p.(?) | P |
| 15 | HOM | c.5221T>C; p.(Ser1741Pro) | LP | c.5221T>C; p.(Ser1741Pro) | LP |
| 16 | HOM | c.2299del; p.(Glu767Serfs*21) | P | c.2299del; p.(Glu767Serfs*21) | P |
| 17 | HET | c.2299del; p.(Glu767Serfs*21) | P | c.4714C>T; p.(Leu1572Phe) | VUS |
| 18 | HET | c.1841-2A>G; p.(?) | P | c.10817T>C; p.(Leu3606Pro) | LP |
| 19 | HET | c.5386T>G; p.(Cys1796Gly) | LP | c.(14998_15131)_(15131_15393)del; p.(?) | P |
| 20 | HET | c.7501C>T; p.(Gln2501*) | P | c.908G>A; p.(Arg303His) | P |
| 21 | HOM | c.9815C>T; p.(Pro3272Leu) | LP | c.9815C>T; p.(Pro3272Leu) | LP |
| 22 | HET | c.13335_13347delinsCTTG; p.(Glu4445_Ser4449delinsAspLeu) | P | c.5153A>C; p.(Gln1718Pro) | VUS |
| 23 | HOM | c.9815C>T; p.(Pro3272Leu) | LP | c.9815C>T; p.(Pro3272Leu) | LP |
| NR | SEX | AGE | ONSET | RE | LE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCVA | VF | EZ | CS | Grade | BCVA | VF | EZ | CS | Grade | |||||
| USH2A | 1 | F | 21 | 8 | 0 | 2 | 3 | 5 | 2 | 0 | 2 | 3 | 5 | 2 |
| 2 | F | 59 | 16 | 5 | 5 | 5 | 15 | 5 | 4 | 5 | 5 | 14 | 5 | |
| 3 | F | 62 | 25 | 5 | 5 | 5 | 15 | 5 | 5 | 5 | 5 | 15 | 5 | |
| 4 | M | 18 | 13 | 1 | 1 | 5 | 7 | 3 | 1 | 1 | 5 | 7 | 3 | |
| 5 | M | 49 | 30 | 3 | 5 | 5 | 13 | 5 | 3 | 5 | 5 | 13 | 5 | |
| 6 | F | 21 | 19 | 3 | 0 | 4 | 7 | 3 | 1 | 0 | 4 | 5 | 2 | |
| 7 | M | 17 | 15 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 2 | 2 | 1 | |
| 8 | M | 48 | 18 | 3 | 5 | 4 | 12 | 4 | 3 | 5 | 5 | 13 | 5 | |
| 9 | F | 27 | 21 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | |
| 10 | M | 32 | 22 | 0 | 0 | 5 | 5 | 2 | 0 | 1 | 5 | 6 | 2 | |
| 11 | M | 31 | 20 | 0 | 1 | 3 | 4 | 2 | 0 | 0 | 4 | 4 | 2 | |
| 12 | M | 55 | 54 | 0 | 1 | 5 | 6 | 2 | 0 | 1 | 5 | 6 | 2 | |
| 13 | F | 36 | 17 | 3 | 1 | 5 | 9 | 3 | 2 | 1 | 5 | 8 | 3 | |
| 14 | M | 62 | 20 | 4 | 5 | 5 | 14 | 5 | 4 | 5 | 5 | 14 | 5 | |
| 15 | F | 47 | 44 | 4 | 5 | 5 | 14 | 5 | 4 | 5 | 5 | 14 | 5 | |
| 16 | F | 30 | 23 | 0 | 3 | 2 | 5 | 2 | 0 | 2 | 2 | 4 | 2 | |
| 17 | M | 42 | 15 | 3 | 5 | 5 | 13 | 5 | 4 | 5 | 5 | 14 | 5 | |
| 18 | M | 55 | 25 | 5 | 3 | 5 | 13 | 5 | 4 | 4 | 5 | 13 | 5 | |
| 19 | F | 59 | 58 | 0 | 2 | 1 | 3 | 1 | 0 | 2 | 1 | 3 | 1 | |
| 20 | M | 72 | 43 | 4 | 2 | 5 | 11 | 4 | 4 | 1 | 5 | 10 | 4 | |
| NSRP | 21 | M | 38 | 37 | 3 | 0 | 4 | 7 | 3 | 4 | 0 | 4 | 8 | 3 |
| 22 | F | 52 | 20 | 3 | 3 | 5 | 11 | 4 | 3 | 3 | 5 | 11 | 4 | |
| 23 | F | 35 | 12 | 1 | 3 | 4 | 8 | 3 | 1 | 2 | 4 | 7 | 3 | |
| N | Sex | Age | Onset | BCVA | VF | EZ | CS | Grade | SRI 5 mm Circle Area (mm2) | SRI Fovea Distance (mm) | Focal ERG (μV) | Submicrovolt 30 Hz (μV) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USH2A | 1 | F | 21 | 8 | 0 | 2 | 3 | 5 | 2 | 0 | 0 | 0.28 | 0.93 |
| 2 | F | 59 | 16 | 5 | 5 | 5 | 15 | 5 | 0 | 2.6 | 0.11 | 0.2 | |
| 3 | F | 62 | 25 | 5 | 5 | 5 | 15 | 5 | 7.2 | 0.3 | 0.11 | 0.07 | |
| 4 | M | 18 | 13 | 1 | 1 | 5 | 7 | 3 | 3.2 | 1.2 | 0.39 | 0.05 | |
| 5 | M | 49 | 30 | 3 | 5 | 5 | 13 | 5 | 0.3 | 1.6 | 0.26 | 0.08 | |
| 6 | F | 21 | 19 | 3 | 0 | 4 | 7 | 3 | 0 | 0 | 0.51 | 2.79 | |
| 7 | M | 17 | 15 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1.07 | 0.73 | |
| 8 | M | 48 | 28 | 3 | 5 | 4 | 12 | 4 | 3.6 | 0.9 | 0.29 | 0.49 | |
| 9 | F | 27 | 21 | 0 | 0 | 1 | 1 | 1 | 0.1 | 2.3 | 1.32 | 1.68 | |
| 10 | M | 32 | 22 | 0 | 0 | 5 | 5 | 2 | 1.8 | 1.7 | 0.27 | 0.69 | |
| 11 | M | 31 | 20 | 0 | 1 | 3 | 4 | 2 | 0 | 2.6 | 0.63 | 0.4 | |
| 12 | M | 55 | 54 | 0 | 1 | 5 | 6 | 2 | 0 | 2.7 | 0.14 | 0.69 | |
| 13 | F | 36 | 17 | 3 | 1 | 5 | 9 | 3 | 0 | 3.6 | 0.12 | 0.69 | |
| 14 | M | 62 | 20 | 4 | 5 | 5 | 14 | 5 | 2.1 | 0.7 | 0.27 | 0.75 | |
| 15 | F | 47 | 44 | 4 | 5 | 5 | 14 | 5 | 1.1 | 1.1 | 0.21 | 0.4 | |
| 16 | F | 30 | 23 | 0 | 3 | 2 | 5 | 2 | 0 | 2.5 | 1.49 | 1.87 | |
| 17 | M | 42 | 15 | 3 | 5 | 5 | 13 | 5 | 2.4 | 0.4 | 0.19 | 0.59 | |
| 18 | M | 55 | 22 | 5 | 3 | 5 | 13 | 5 | 2.8 | 0.5 | 0.1 | 0.59 | |
| 19 | F | 59 | 58 | 0 | 2 | 1 | 3 | 1 | 0 | 0 | 1.99 | 1.93 | |
| 20 | M | 72 | 43 | 4 | 2 | 5 | 11 | 4 | 9.8 | 0.3 | 0.12 | 2.17 | |
| NSRP | 21 | M | 38 | 37 | 3 | 0 | 4 | 7 | 3 | 0 | 0 | 0.23 | 0.96 |
| 22 | F | 52 | 20 | 3 | 3 | 5 | 11 | 4 | 0.2 | 2 | 0.15 | 0.38 | |
| 23 | F | 35 | 12 | 1 | 3 | 4 | 8 | 3 | 0 | 3.5 | 0.12 | 0.79 |
| Rho | p-Value | |
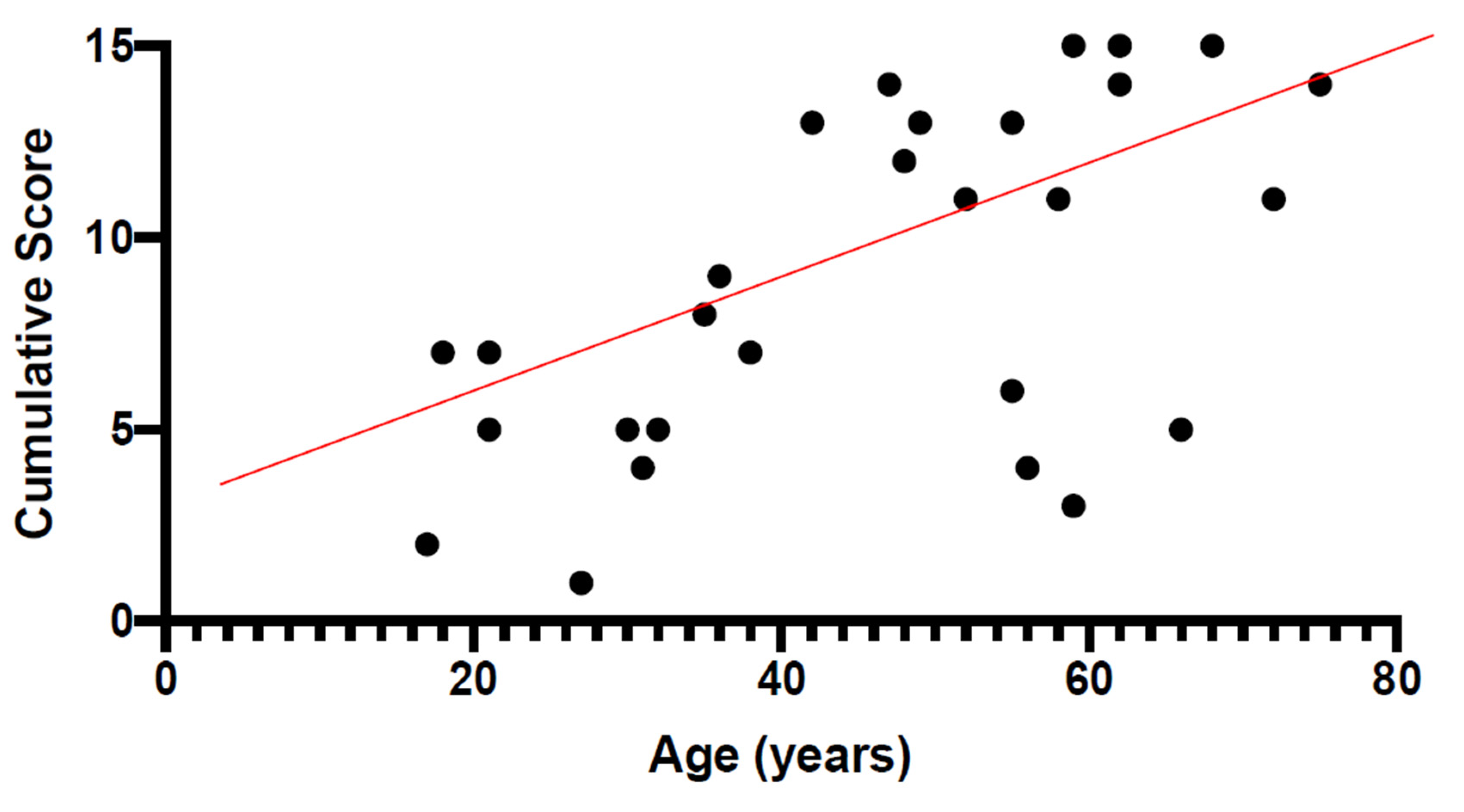
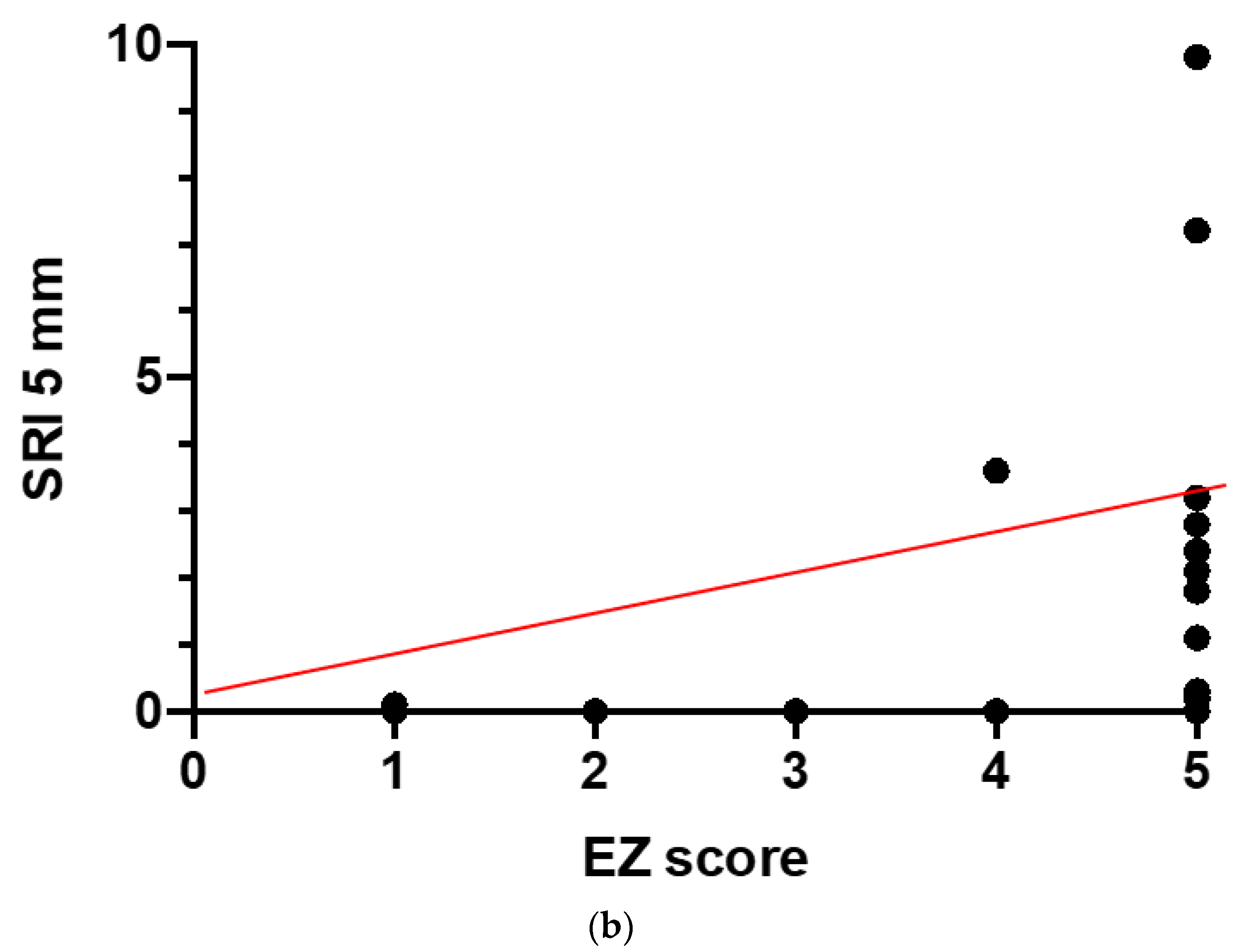
|---|---|---|
| FERG vs. CS | −0.72 | <0.0001 |
| smVolt vs. CS | −0.58 | 0.004 |
| SRI vs. CS | 0.53 | 0.01 |
| SRI vs. EZ | 0.54 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falsini, B.; Placidi, G.; De Siena, E.; Savastano, M.C.; Minnella, A.M.; Maceroni, M.; Midena, G.; Ziccardi, L.; Parisi, V.; Bertelli, M.; et al. USH2A-Related Retinitis Pigmentosa: Staging of Disease Severity and Morpho-Functional Studies. Diagnostics 2021, 11, 213. https://doi.org/10.3390/diagnostics11020213
Falsini B, Placidi G, De Siena E, Savastano MC, Minnella AM, Maceroni M, Midena G, Ziccardi L, Parisi V, Bertelli M, et al. USH2A-Related Retinitis Pigmentosa: Staging of Disease Severity and Morpho-Functional Studies. Diagnostics. 2021; 11(2):213. https://doi.org/10.3390/diagnostics11020213
Chicago/Turabian StyleFalsini, Benedetto, Giorgio Placidi, Elisa De Siena, Maria Cristina Savastano, Angelo Maria Minnella, Martina Maceroni, Giulia Midena, Lucia Ziccardi, Vincenzo Parisi, Matteo Bertelli, and et al. 2021. "USH2A-Related Retinitis Pigmentosa: Staging of Disease Severity and Morpho-Functional Studies" Diagnostics 11, no. 2: 213. https://doi.org/10.3390/diagnostics11020213
APA StyleFalsini, B., Placidi, G., De Siena, E., Savastano, M. C., Minnella, A. M., Maceroni, M., Midena, G., Ziccardi, L., Parisi, V., Bertelli, M., Maltese, P. E., Chiurazzi, P., & Rizzo, S. (2021). USH2A-Related Retinitis Pigmentosa: Staging of Disease Severity and Morpho-Functional Studies. Diagnostics, 11(2), 213. https://doi.org/10.3390/diagnostics11020213










