Dual-Energy Computed Tomography of the Liver: Uses in Clinical Practices and Applications
Abstract
:1. Introduction
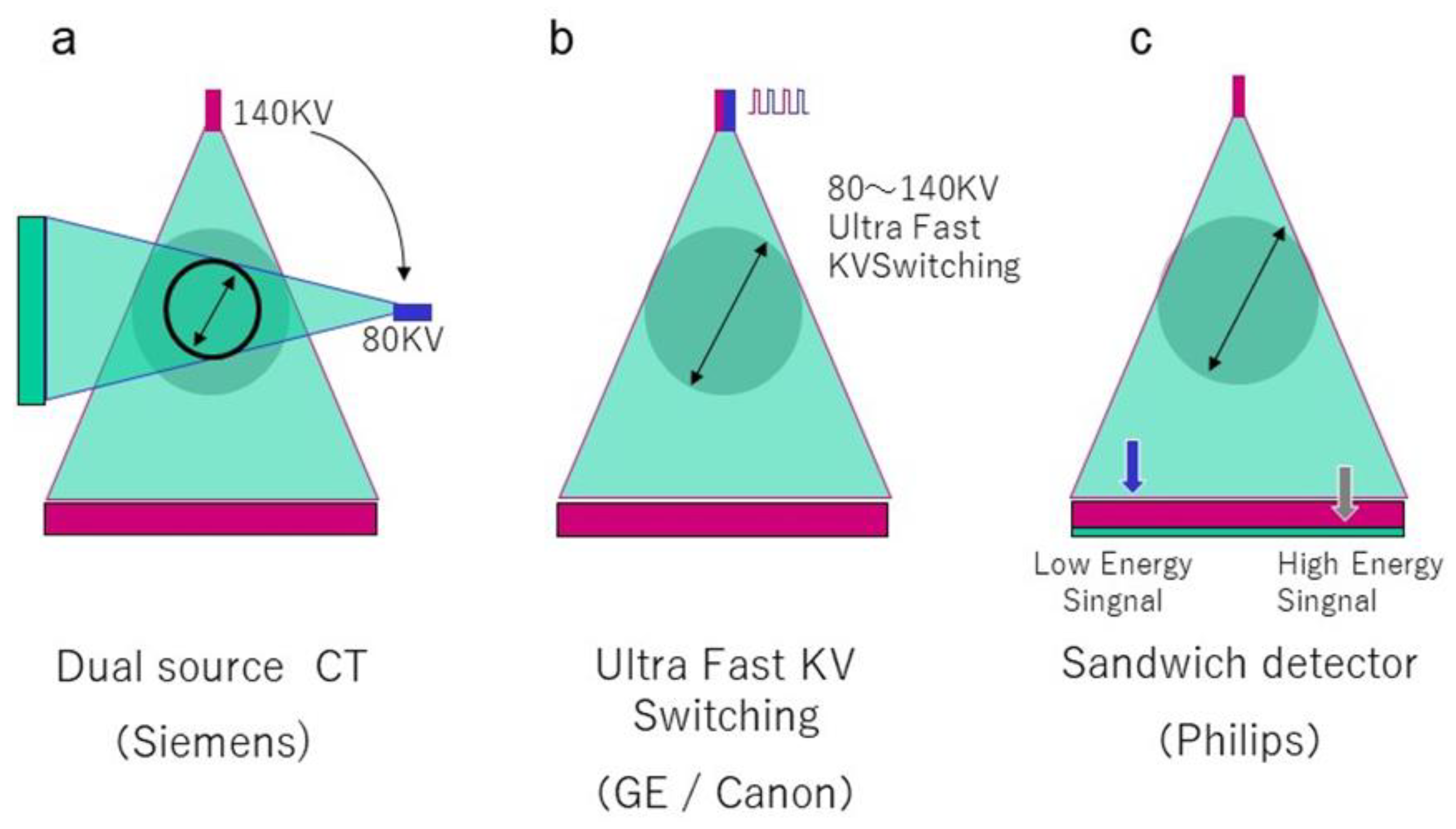
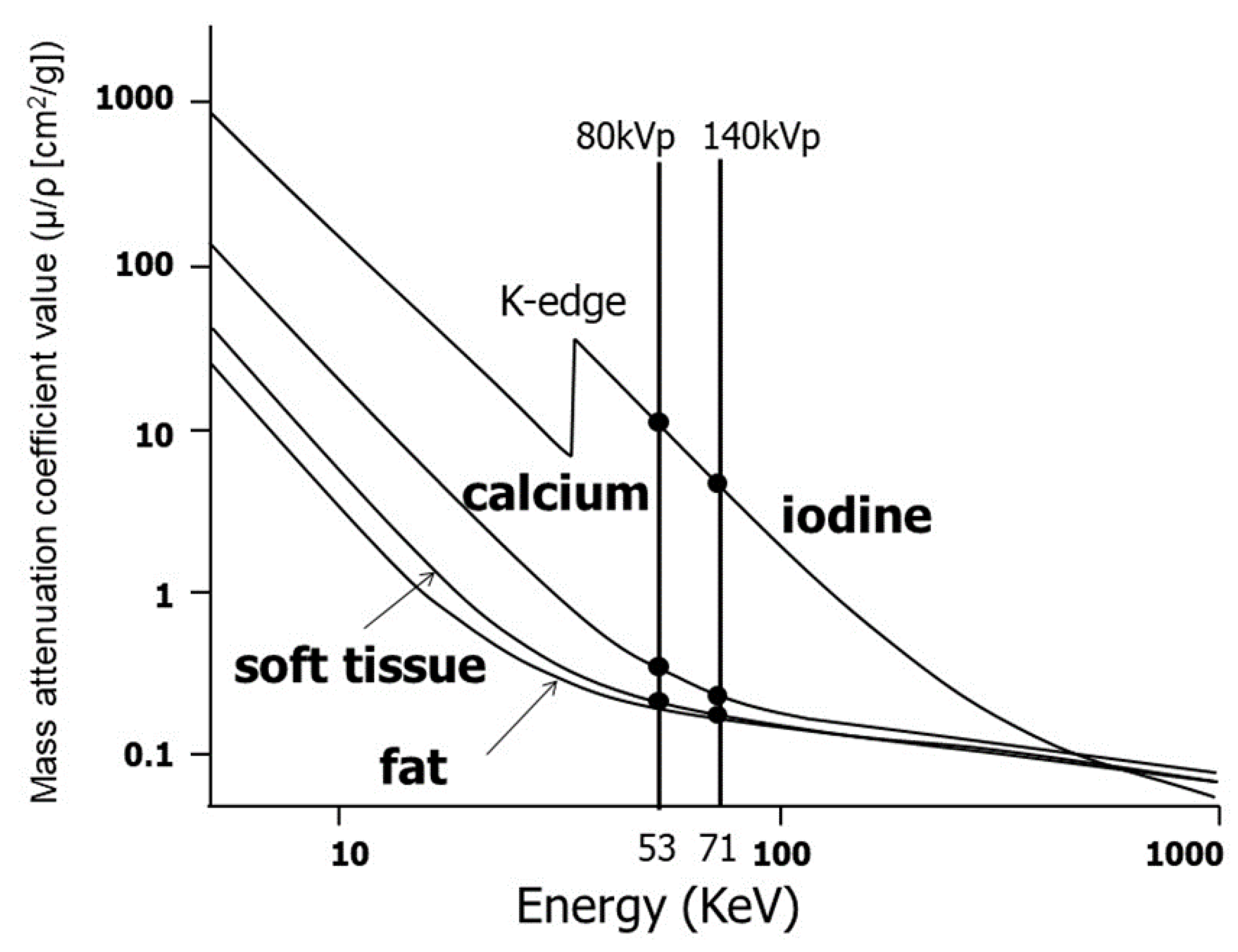
2. Recent Developments and Basic Principles of DECT
3. Information Obtained through Dual-Energy Imaging and Its Clinical Applications
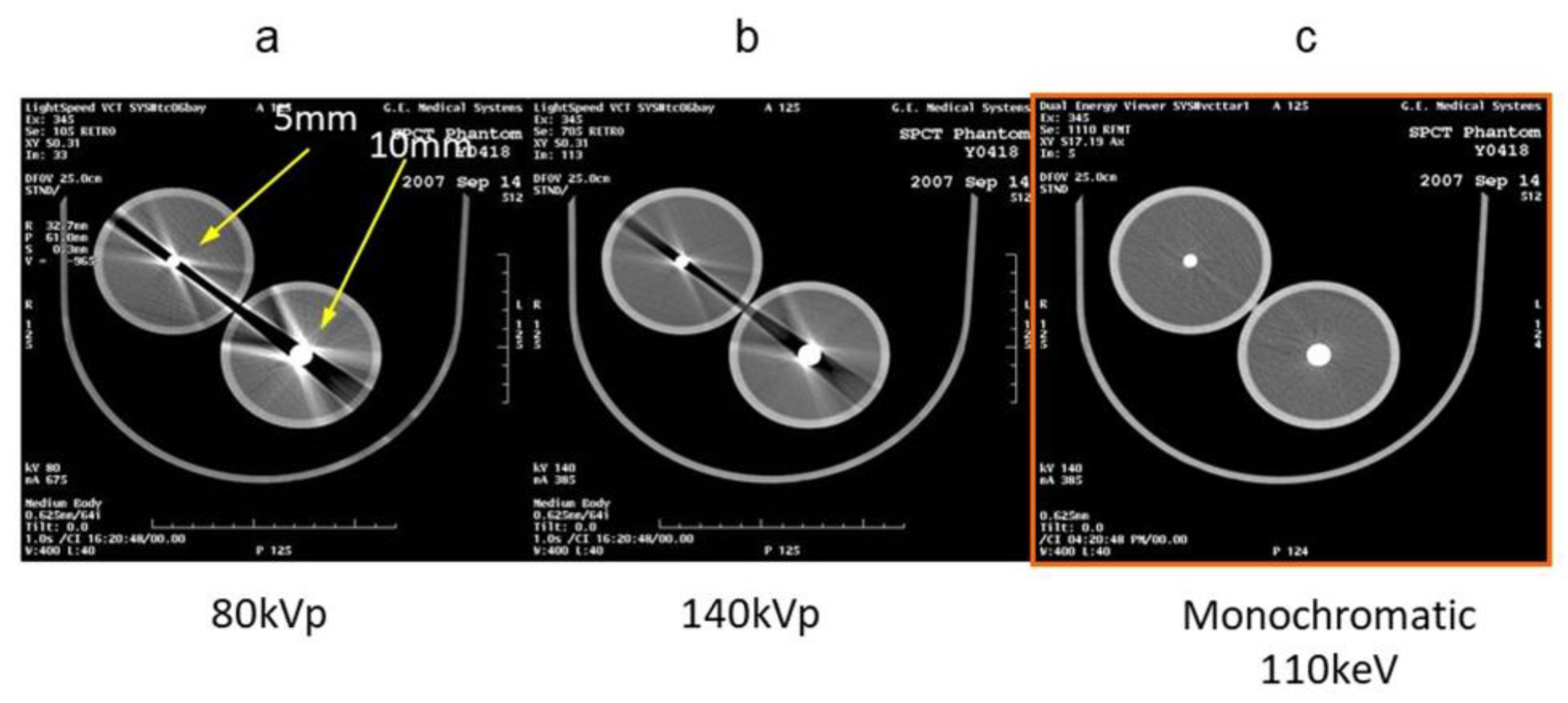
3.1. Virtual Monochromatic Imaging
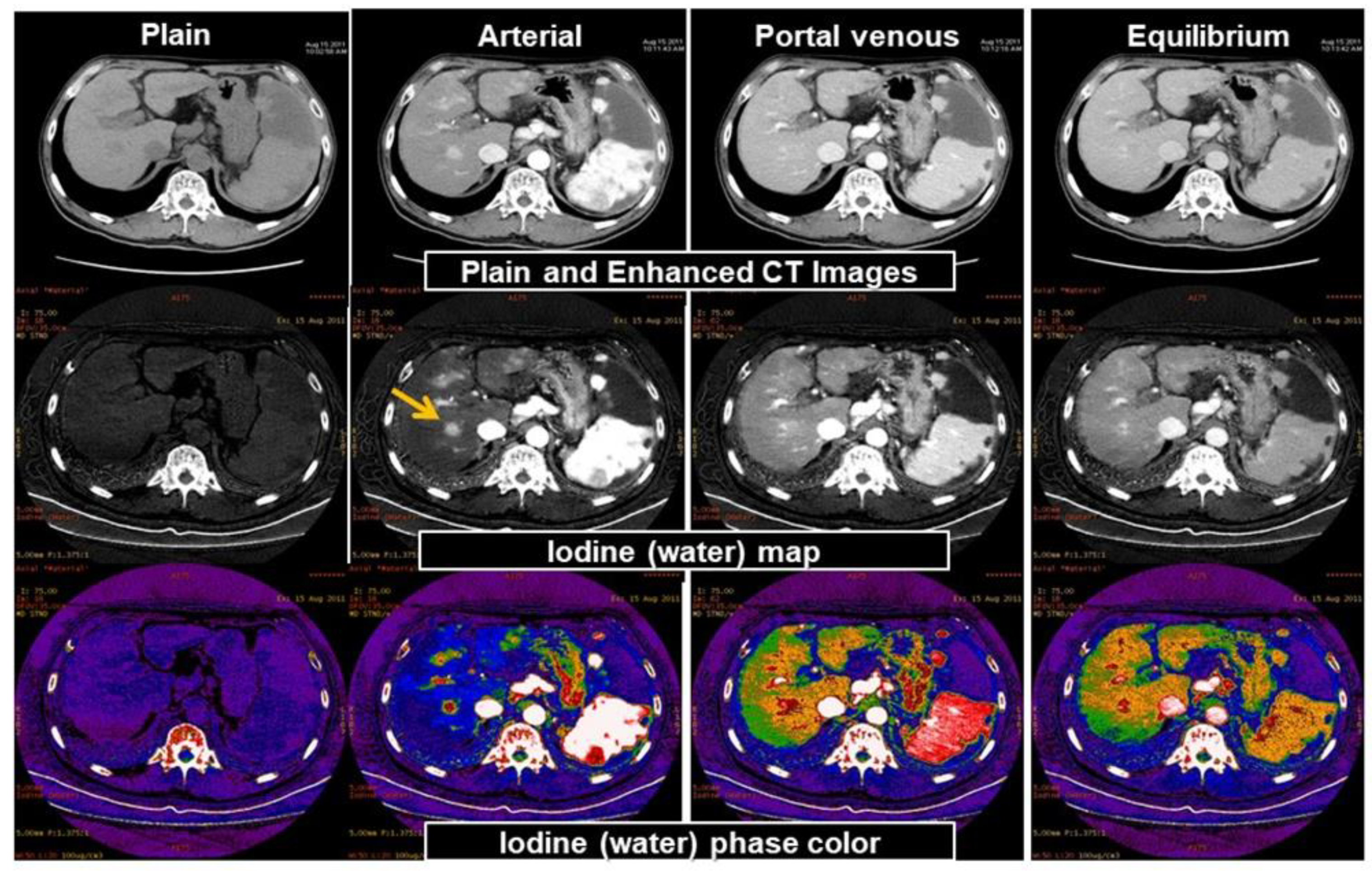
3.2. Material Decomposition
3.3. Virtual Unenhanced CT
3.4. Limitations
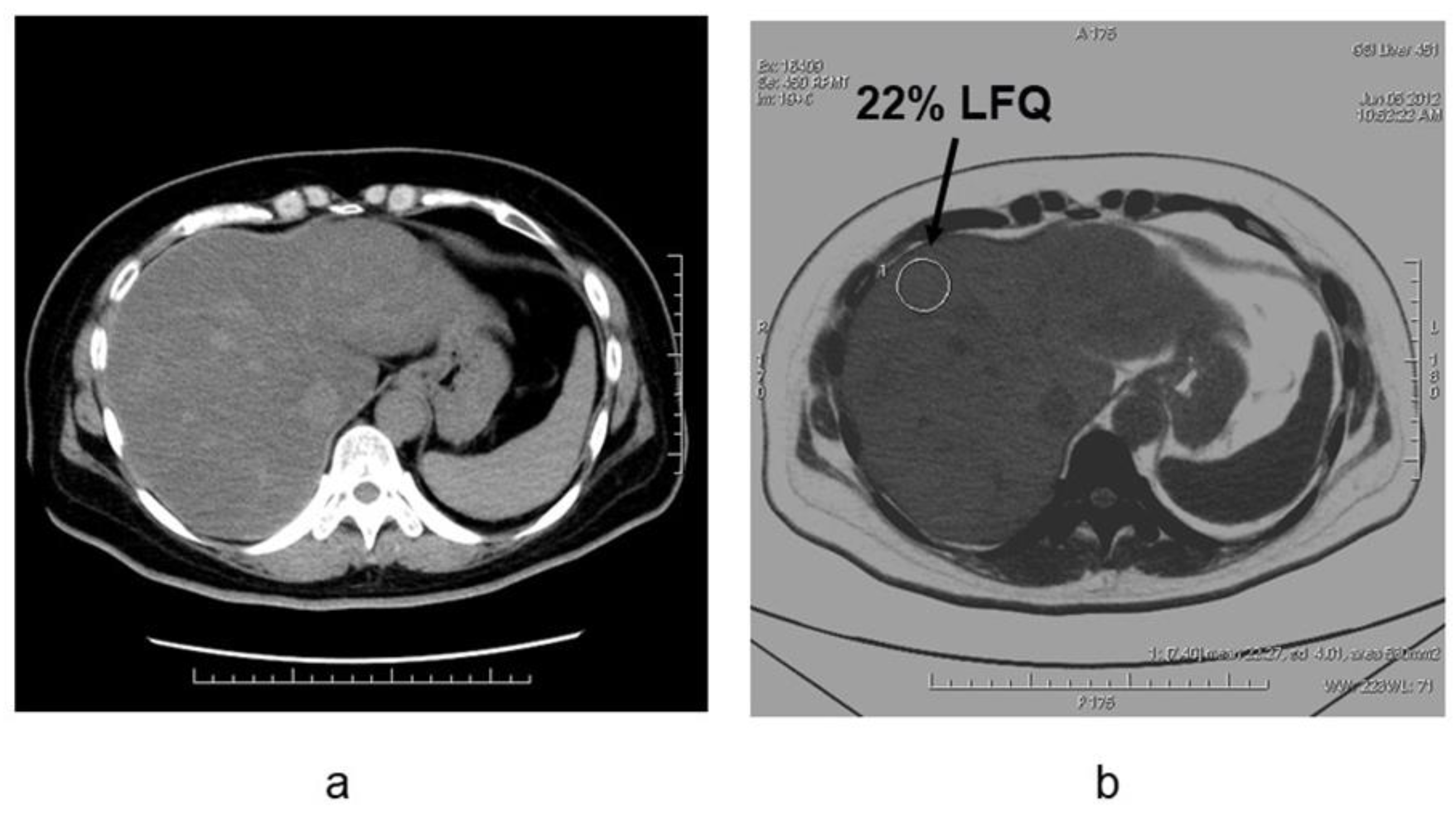
4. Evaluation of Hepatic Steatosis Using DECT
5. Estimation of Hepatic Fibrosis Using DECT
6. Potential Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Flohr, T.G.; McCollough, C.H.; Bruder, H.; Petersilka, M.; Gruber, K.; Süss, C.; Grasruck, M.; Stierstorfer, K.; Krauss, B.; Raupach, R.; et al. First performance evaluation of a dual-source CT (DSCT) system. Eur. Radiol. 2006, 16, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; McCollough, C. Dual-energy computed tomography. In Computed Tomography of the Cardiovascular System; Gerber, T.C., Kantor, B., Williamson, E.E., Eds.; Informa Healthcare: London, UK, 2007; pp. 451–462. [Google Scholar]
- Roos, B.O. Dual photon absorptiometry in lumbar vertebrae: One theory and method. Acta. Radiol. Ther. Phys. Biol. 1974, 13, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.T.; Sones, R.A.; Tesic, M.M.; Morgan, D.R.; Sanders, J.N. Detector for Dual-Energy Digital Radiography. Radiology 1985, 156, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Hickey, N.M.; Niklason, L.T.; Sabbagh, E.; Fraser, R.G.; Barnes, G.T. Dual-Energy Digital Radiographic Quantification of Calcium in Simulated Pulmonary Nodules Radiology. Am. J. Reontgenol. 1987, 148, 19–24. [Google Scholar] [CrossRef]
- Friedman, S.E.; Dubovsky, E.V.; Dubovsky, J.; Alexander, C.B.; Robinson, C.A.; Sabbagh, E.A.; Barnes, G.T.; Fraser, R.G. Mineral Content of Bone Measurement by Energy Subtraction Digital Chest Radiography. Am. J. Reontgenol. 1987, 149, 1119–1202. [Google Scholar] [CrossRef]
- Sartoris, D.J.; Resnick, D. Dual-Energy Radiographic Absorptiometry for Bone Densitometry; Current Status and Perspective. Am. J. Reontgenol. 1989, 152, 241–246. [Google Scholar] [CrossRef]
- Gundry, C.R.; Miller, C.W.; Ramos, E.; Moscona, A.; Stein, J.A.; Mazess, R.B.; Sartoris, D.J.; Resnick, D. Dual-Energy Radiographic Absorptiometry of the Lumbar Spine: Clinical Experience with Two Different Systems. Radiology 1990, 174, 539–541. [Google Scholar] [CrossRef]
- Joe, E.; Kim, S.H.; Lee, K.B.; Jang, J.J.; Lee, J.Y.; Lee, J.M.; Han, J.K.; Choi, B.I. Feasibility and accuracy of dual-source dual-energy CT for noninvasive determination of hepatic iron accumulation. Radiology 2012, 262, 126–135. [Google Scholar] [CrossRef]
- Fink, C. Liver imaging. In Dual Energy CT in Clinical Practice. Medical Radiology; Johnson, T., Fink, C., Schonberg, S.O., Reiser, M.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 145–155. [Google Scholar]
- Fang, T.; Deng, W.; Law, M.W.; Luo, L.; Zheng, L.; Guo, Y.; Chen, H.; Huang, B. Comparison of image quality and radiation exposure between conventional imaging and gemstone spectral imaging in abdominal CT examination. Br. J. Radiol. 2018, 91, 20170448. [Google Scholar] [CrossRef]
- Wichmann, J.L.; Hardie, A.D.; Schoepf, U.J.; Felmly, L.M.; Perry, J.D.; Varga-Szemes, A.; Mangold, S.; Caruso, D.; Canstein, C.; Vogl, T.J.; et al. Single- and dual-energy CT of the ab-domen: Comparison of radiation dose and image quality of 2nd and 3rd generation dual-source CT. Eur. Radiol. 2017, 27, 642–650. [Google Scholar] [CrossRef]
- Uhrig, M.; Simons, D.; Kachelriess, M.; Pisana, F.; Kuchenbecker, S.; Schlemmer, H.P. Advanced abdominal imaging with dual energy CT is feasible without increasing radiation dose. Cancer Imaging 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, T.D.; Rheinheimer, S.; Kauczor, H.U.; Stiller, W.; Weber, T.; Skornitzke, S. Image quality evaluation of dual-layer spectral CT in comparison to single-layer CT in a reduced-dose setting. Eur. Radiol. 2020, 30, 5709–5719. [Google Scholar] [CrossRef] [PubMed]
- Yeh, B.M.; Shepherd, J.A.; Wang, Z.J.; Teh, H.S.; Hartman, R.P.; Prevrhal, S. Dual-energy and low-kVp CT in the abdomen. AJR Am. J. Roentgenol. 2009, 193, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.R.; Krauss, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Morse, B.G.; Hara, A.K.; Paden, R.G.; Hongo, N.; Pavlicek, W. Dual-energy (spectral) CT: Applications in abdominal imaging. Radiographics 2011, 31, 1031–1046. [Google Scholar] [CrossRef]
- Hidas, G.; Eliahou, R.; Duvdevani, M.; Coulon, P.; Lemaitre, L.; Gofrit, O.N.; Pode, D.; Sosna, J. Determination of renal stone composition with dual-energy CT: In vivo analysis and comparison with X-ray diffraction. Radiology 2010, 257, 394–401. [Google Scholar] [CrossRef]
- Avrin, D.E.; Macovski, A.; Zatz, L.E. Clinical application of Compton and photo-electric reconstruction in computed tomography: Preliminary results. Invest Radiol. 1978, 13, 217–222. [Google Scholar] [CrossRef]
- Coursey, C.A.; Nelson, R.C.; Boll, D.T.; Paulson, E.K.; Ho, L.M.; Neville, A.M.; Marin, D.; Gupta, R.T.; Schindera, S.T. Dual-energy multidetector CT: How does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics 2010, 30, 1037–1055. [Google Scholar] [CrossRef]
- McCollough, C.; Schmidt, B.; Liu, X.; Yu, L.; Leng, S. Dual-Energy Algorithms and Postprocessing Techniques. In Dual Energy CT in Clinical Practice; Johnson, T.R.C., Fink, C., Schoenberg, S.O., Reiser, M.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 43–51. [Google Scholar]
- Matsumoto, K.; Jinzaki, M.; Tanami, Y.; Ueno, A.; Yamada, M.; Kuribayashi, S. Virtual monochromatic spectral imaging with fast kilovoltage switching; Improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 2011, 259, 257–262. [Google Scholar] [CrossRef]
- Mileto, A.; Nelson, R.C.; Marin, D.; Roy Choudhury, K.; Ho, L.M. Dual-Energy Multidetector CT for the Characterization of Incidental Adrenal Nodules; Diagnostic Performance of Contrastenhanced Material Density Analysis. Radiology 2015, 274, 445–454. [Google Scholar] [CrossRef]
- Shinohara, Y.; Sakamoto, M.; Kuya, K.; Kishimoto, J.; Iwata, N.; Ohta, Y.; Fujii, S.; Watanabe, T.; Ogawa, T. Assessment of carotid plaque composition using fast-kV switching dual-energy CT with gemstone detector: Comparison with extracorporeal and virtual histology-intravascular ultrasound. Neuroradiology 2015, 57, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Hirose, K.; Sato, M.; Akimoto, H.; Kawaguchi, H.; Hatayama, Y.; Fujioka, I.; Tanaka, M.; Ono, S.; Takai, Y. Prognostic impact of average iodine density assessed by dual-energy spectral imaging for predicting lung tumor recurrence after stereotactic body radiotherapy. J. Radiat. Res. 2016, 57, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Abdel Razek, A.A.K.; El-Serougy, L.G.; Saleh, G.A.; Shabana, W.; Abd El-Wahab, R. Liver Imaging Reporting and Data System Version 2018: What Radiologists Need to Know. J. Comput. Assist Tomogr. 2020, 44, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Corrias, G.; Sawan, P.; Mahmood, U.; Zheng, J.; Capanu, M.; Salvatore, M.; Spinato, G.; Saba, L.; Mannelli, L. Dual energy computed tomography analysis in cancer patients: What factors affect iodine concentration in contrast enhanced studies? Eur. J. Radiol. 2019, 120, 108698. [Google Scholar] [CrossRef]
- Yamada, Y.; Jinzaki, M.; Tanami, Y.; Abe, T.; Kuribayashi, S. Virtual monochromatic spectral imaging for the evaluation of hypovascular hepatic metastases: The optimal monochromatic level with fast kilovoltage switching dual-energy computed tomography. Investig. Radiol. 2012, 47, 292–298. [Google Scholar] [CrossRef]
- Caruso, D.; De Cecco, C.N.; Schoepf, U.J.; Schaefer, A.R.; Leland, P.W.; Johnson, D.; Laghi, A.; Hardie, A.D. Can dual-energy computed tomography improve visualization of hypoenhancing liver lesions in portal venous phase? Assessment of advanced image-based virtual monoenergetic images. Clin. Imaging 2017, 41, 118–124. [Google Scholar] [CrossRef]
- Nyman, U.; Björk, J.; Aspelin, P.; Marenzi, G. Contrast medium dose-to-GFR ratio: A measure of systemic exposure to predict contrast induced nephropathy after percutaneous coronary intervention. Acta Radiol. 2008, 49, 658–667. [Google Scholar] [CrossRef]
- Nakayama, Y.; Awai, K.; Funama, Y.; Liu, D.; Nakaura, T.; Tamura, Y.; Yamashita, Y. Lower tube voltage reduces contrast material and radiation doses on 16-MDCT aortography. AJR Am. J. Roentgenol. 2006, 187, W490–W497. [Google Scholar] [CrossRef]
- Lv, P.; Lin, X.Z.; Chen, K.; Gao, J. Spectral CT in patients with small HCC: Investigation of image quality and diagnostic accuracy. Eur. Radiol. 2012, 22, 2117–2124. [Google Scholar] [CrossRef]
- Liu, Y.S.; Chuang, M.T.; Tsai, Y.S.; Tsai, H.M.; Lin, X.Z. Nitroglycerine use in transcatheter arterial (chemo) embolization in patients with hepatocellular carcinoma and dual-energy CT assessment of Lipiodol retention. Eur. Radiol. 2012, 22, 2193–2200. [Google Scholar] [CrossRef]
- Zhang, L.J.; Peng, J.; Wu, S.Y.; Wang, Z.J.; Wu, X.S.; Zhou, C.S.; Ji, X.M.; Lu, G.M. Liver virtual non-enhanced CT with dual-source, dual-energy CT: A preliminary study. Eur. Radiol. 2010, 20, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Horvat, N.; Horvat, J.V.; Ryan, D.; Gao, Y.; Carollo, G.; DeOcampo, R.; Do, R.K.; Katz, S.; Gerst, S.; et al. Rapid switching kVp dual energy CT: Value of reconstructed dual energy CT images and organ dose assessment in multiphasic liver CT exams. Eur. J. Radiol. 2018, 102, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bohte, A.E.; van Werven, J.R.; Bipat, S.; Stoker, J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: A meta-analysis. Eur. Radiol. 2011, 21, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühn, J.P.; Hernando, D.; Mensel, B.; Krüger, P.C.; Ittermann, T.; Mayerle, J.; Hosten, N.; Reeder, S.B. Quantitative chemical shift-encoded MRI is an accurate method to quantify hepatic steatosis. J. Magn. Reson. Imaging 2014, 39, 1494–1501. [Google Scholar] [CrossRef] [Green Version]
- Kühn, J.P.; Hernando, D.; Muñoz del Rio, A.; Evert, M.; Kannengiesser, S.; Völzke, H.; Mensel, B.; Puls, R.; Hosten, N.; Reeder, S.B. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: Correlation of biopsy with MR imaging results. Radiology 2012, 265, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Awai, H.I.; Newton, K.P.; Sirlin, C.B.; Behling, C.; Schwimmer, J.B. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin. Gastroenterol. Hepatol. 2014, 12, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.S.; Park, S.H.; Lee, S.S.; Kim, D.Y.; Shin, Y.M.; Lee, W.; Lee, S.G.; Yu, E.S. Biopsy-proven nonsteatotic liver in adults: Estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology 2011, 258, 760–766. [Google Scholar] [CrossRef]
- Hyodo, T.; Yada, N.; Hori, M.; Maenishi, O.; Lamb, P.; Sasaki, K.; Onoda, M.; Kudo, M.; Mochizuki, T.; Murakami, T. Multimaterial Decomposition Algorithm for the Quantification of Liver Fat Content by Using Fast-Kilovolt-Peak Switching Dual-Energy CT: Clinical Evaluation. Radiology 2017, 283, 108–118. [Google Scholar] [CrossRef]
- Hyodo, T.; Hori, M.; Lamb, P.; Sasaki, K.; Wakayama, T.; Chiba, Y.; Mochizuki, T.; Murakami, T. Multimaterial Decomposition Algorithm for the Quantification of Liver Fat Content by Using Fast-Kilovolt-Peak Switching Dual-Energy CT: Experimental Validation. Radiology 2017, 282, 381–389. [Google Scholar] [CrossRef]
- Fischer, M.A.; Gnannt, R.; Raptis, D.; Reiner, C.S.; Clavien, P.A.; Schmidt, B.; Leschka, S.; Alkadhi, H.; Goetti, R. Quantification of liver fat in the presence of iron and iodine: An ex-vivo dual-energy CT study. Investig. Radiol. 2011, 46, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Hur, B.Y.; Lee, J.M.; Hyunsik, W.; Lee, K.B.; Joo, I.; Han, J.K.; Choi, B.I. Quantification of the fat fraction in the liver using dual-energy computed tomography and multimaterial decomposition. J. Comput. Assist Tomogr. 2014, 38, 845–852. [Google Scholar] [CrossRef]
- Mendonca, P.R.; Lamb, P.; Sahani, D.V. A Flexible Method for Multi-Material Decomposition of Dual-Energy CT Images. IEEE Trans. Med. Imaging 2014, 33, 99–116. [Google Scholar] [CrossRef]
- Horowitz, J.M.; Venkatesh, S.K.; Ehman, R.L.; Jhaveri, K.; Kamath, P.; Ohliger, M.A.; Samir, A.E.; Silva, A.C.; Taouli, B.; Torbenson, M.S.; et al. Evaluation of hepatic fibrosis: A review from the society of abdominal radiology disease focus panel. Abdom. Radiol. 2017, 42, 2037–2053. [Google Scholar] [CrossRef]
- Barr, R.G. Shear wave liver elastography. Abdom. Radiol. 2018, 43, 800–807. [Google Scholar] [CrossRef]
- Kennedy, P.; Wagner, M.; Castéra, L.; Hong, C.W.; Johnson, C.L.; Sirlin, C.B.; Taouli, B. Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology 2018, 286, 738–763. [Google Scholar] [CrossRef]
- Razek, A.A.; Khashaba, M.; Abdalla, A.; Bayomy, M.; Barakat, T. Apparent diffusion coefficient value of hepatic fibrosis and inflammation in children with chronic hepatitis. Radiol. Med. 2014, 119, 903–909. [Google Scholar] [CrossRef]
- Besheer, T.; Elalfy, H.; Abd El-Maksoud, M.; Abd El-Razek, A.; Taman, S.; Zalata, K.; Elkashef, W.; Zaghloul, H.; Elshahawy, H.; Raafat, D.; et al. Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus. World J. Gastroenterol. 2019, 25, 1366–1377. [Google Scholar] [CrossRef]
- Varenika, V.; Fu, Y.; Maher, J.J.; Gao, D.; Kakar, S.; Cabarrus, M.C.; Yeh, B.M. Hepatic fibrosis: Evaluation with semiquantitative contrast-enhanced CT. Radiology 2013, 266, 151–158. [Google Scholar] [CrossRef]
- Zissen, M.H.; Wang, Z.J.; Yee, J.; Aslam, R.; Monto, A.; Yeh, B.M. Contrast-enhanced CT quantification of the hepatic fractional extracellular space: Correlation with diffuse liver disease severity. Am. J. Reontgenol. 2013, 201, 1204–1210. [Google Scholar] [CrossRef] [Green Version]
- Bandula, S.; Punwani, S.; Rosenberg, W.M.; Jalan, R.; Hall, A.R.; Dhillon, A.; Moon, J.C.; Taylor, S.A. Equilibrium contrast-enhanced CT imaging to evaluate hepatic fibrosis: Initial validation by comparison with histopathologic sampling. Radiology 2015, 275, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.H.; Lee, J.M.; Klotz, E.; Jeon, J.H.; Lee, K.B.; Han, J.K.; Choi, B.I. Estimation of hepatic extracellular volume fraction using multiphasic liver computed tomography for hepatic fibrosis grading. Investig. Radiol. 2015, 50, 290–296. [Google Scholar] [CrossRef]
- Guo, S.L.; Su, L.N.; Zhai, Y.N.; Chirume, W.M.; Lei, J.Q.; Zhang, H.; Yang, L.; Shen, X.P.; Wen, X.X.; Guo, Y.M. The clinical value of hepatic extracellular volume fraction using routine multiphasic contrast-enhanced liver CT for staging liver fibrosis. Clin. Radiol. 2017, 72, 242–246. [Google Scholar] [CrossRef]
- Shinagawa, Y.; Sakamoto, K.; Sato, K.; Ito, E.; Urakawa, H.; Yoshimitsu, K. Usefulness of new subtraction algorithm in estimating degree of liver fibrosis by calculating extracellular volume fraction obtained from routine liver CT protocol equilibrium phase data: Preliminary experience. Eur. J. Radiol. 2018, 103, 99–104. [Google Scholar] [CrossRef]
- Sofue, K.; Tsurusaki, M.; Mileto, A.; Hyodo, T.; Sasaki, K.; Nishii, T.; Chikugo, T.; Yada, N.; Kudo, M.; Sugimura, K.; et al. Dual-energy computed tomography for non-invasive staging of liver fibrosis: Accuracy of iodine density measurements from contrast-enhanced data. Hepatol. Res. 2018, 48, 1008–1019. [Google Scholar] [CrossRef]
- Yu, L.; Primak, A.N.; Liu, X.; McCollough, C.H. Image quality optimization and evaluation of linearly mixed images in dual-source, dual-energy CT. Med. Phys. 2009, 36, 1019–1024. [Google Scholar] [CrossRef]
- Vrtiska, T.J.; Takahashi, N.; Fletcher, J.G.; Hartman, R.P.; Yu, L.; Kawashima, A. Genitourinary applications of dual-energy CT. AJR Am. J. Roentgenol. 2010, 194, 1434–1442. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurusaki, M.; Sofue, K.; Hori, M.; Sasaki, K.; Ishii, K.; Murakami, T.; Kudo, M. Dual-Energy Computed Tomography of the Liver: Uses in Clinical Practices and Applications. Diagnostics 2021, 11, 161. https://doi.org/10.3390/diagnostics11020161
Tsurusaki M, Sofue K, Hori M, Sasaki K, Ishii K, Murakami T, Kudo M. Dual-Energy Computed Tomography of the Liver: Uses in Clinical Practices and Applications. Diagnostics. 2021; 11(2):161. https://doi.org/10.3390/diagnostics11020161
Chicago/Turabian StyleTsurusaki, Masakatsu, Keitaro Sofue, Masatoshi Hori, Kosuke Sasaki, Kazunari Ishii, Takamichi Murakami, and Masatoshi Kudo. 2021. "Dual-Energy Computed Tomography of the Liver: Uses in Clinical Practices and Applications" Diagnostics 11, no. 2: 161. https://doi.org/10.3390/diagnostics11020161
APA StyleTsurusaki, M., Sofue, K., Hori, M., Sasaki, K., Ishii, K., Murakami, T., & Kudo, M. (2021). Dual-Energy Computed Tomography of the Liver: Uses in Clinical Practices and Applications. Diagnostics, 11(2), 161. https://doi.org/10.3390/diagnostics11020161







