The Summarized Assessment of Endothelin A Receptor Expression in Renal Transplant Compartments Associated with Antibody-Mediated Rejection
Abstract
:1. Introduction
2. Methods
2.1. Participants and Settings
2.2. Data Analysis
3. Results
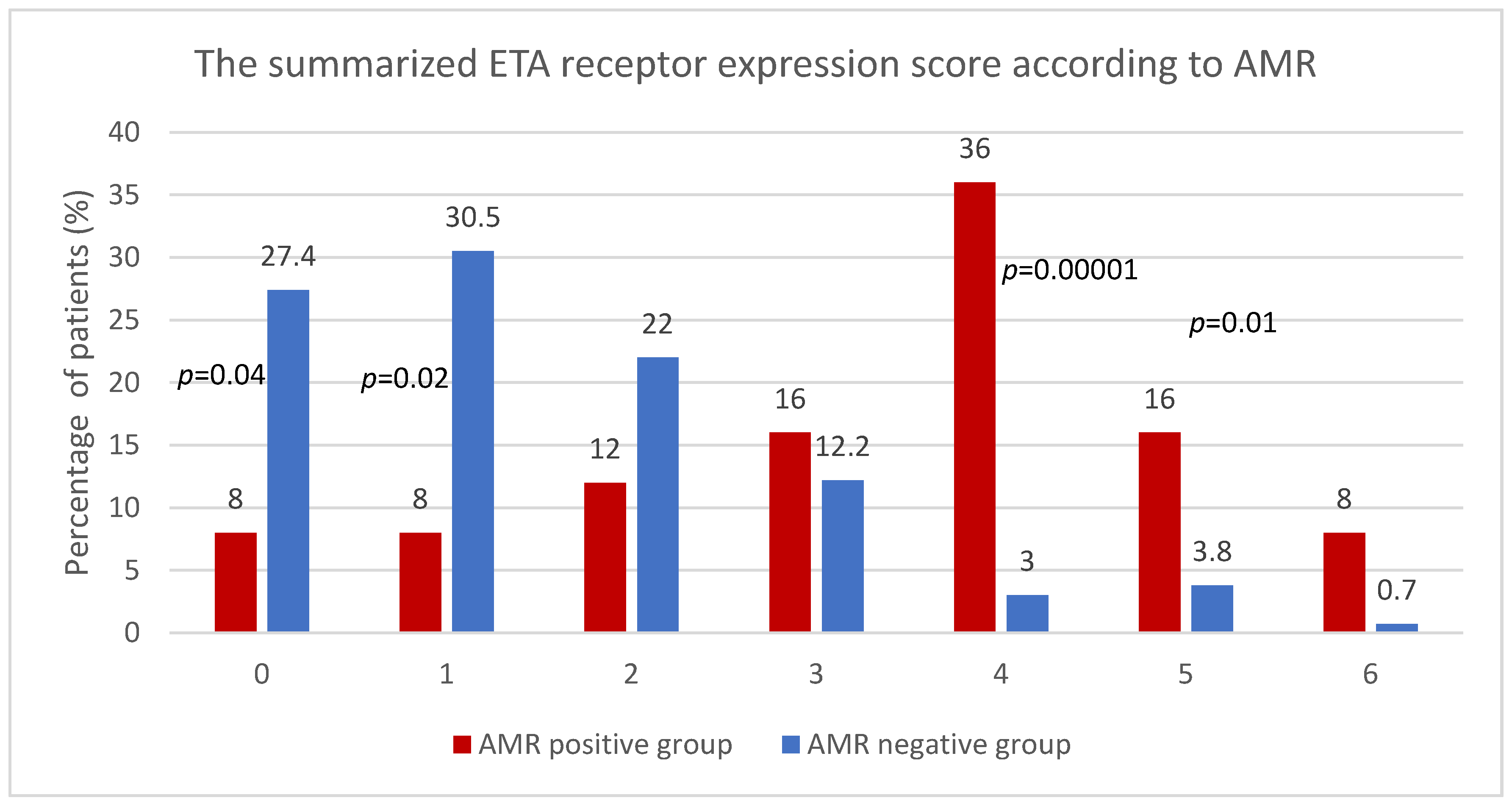
3.1. The Summarized ETA Receptor Expression Score
3.2. The Summarized ETA Receptor Expression Score and AMR
3.3. The Mean Summarized ETA Receptor Expression in AMR Positive and AMR Negative Group
3.4. ROC Analysis of ETAR for Detecting AMR Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AMR | antibody-mediated rejection: |
| ATN | acute tubular necrosis; |
| AT1 receptor | angiotensin II type 1 receptor; |
| anti-AT1R antibodies | angiotensin type 1-receptor antibodies; |
| anti-ETAR antibodies | endothelin A receptor antibodies; |
| DSA | donor-specific antibody; |
| ET | endothelin; |
| ERK | extracellular signal-regulated kinase; |
| ETA receptor | endothelin A receptor; |
| GPCR | G-protein-coupled receptor; |
| HLA | human leukocyte antibody; |
| IVIG | intravenous immunoglobulin; |
| OR | odds ratio; |
| PRA | panel reactive antibody. |
References
- Terasaki, P.I. Humoral theory of transplantation. Am. J. Transplant. 2003, 3, 665–673. [Google Scholar] [CrossRef]
- Sellares, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C. Immune response to non-HLA antigens and renal allograft loss. Lancet 2019, 393, 854–856. [Google Scholar] [CrossRef]
- Dragun, D.; Hegner, B. Non-HLA antibodies post-transplantation: Clinical relevance and treatment in solid organ transplantation. Contrib. Nephrol. 2009, 162, 129–139. [Google Scholar] [CrossRef]
- Lefaucheur, C.; Viglietti, D.; Bouatou, Y.; Philippe, A.; Pievani, D.; Aubert, O.; Duong Van Huyen, J.P.; Taupin, J.L.; Glotz, D.; Legendre, C.; et al. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. 2019, 96, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Banasik, M.; Boratynska, M.; Nowakowska, B.; Halon, A.; Koscielska-Kasprzak, K.; Drulis-Fajdasz, D.; Patrzalek, D.; Weyde, W.; Klinger, M. Variability in donor-specific alloantibody production after transplantation. Transplant. Proc. 2007, 39, 2715–2717. [Google Scholar] [CrossRef]
- Banasik, M.; Boratynska, M.; Koscielska-Kasprzak, K.; Kaminska, D.; Bartoszek, D.; Zabinska, M.; Myszka, M.; Zmonarski, S.; Protasiewicz, M.; Nowakowska, B.; et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl. Int. 2014, 27, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet 2005, 365, 1570–1576. [Google Scholar] [CrossRef]
- Crespo, M.; Llinàs-Mallol, L.; Redondo-Pachón, D.; Butler, C.; Gimeno, J.; Pérez-Sáez, M.J.; Burballa, C.; Buxeda, A.; Arias-Cabrales, C.; Folgueiras, M.; et al. Non-HLA Antibodies and Epitope Mismatches in Kidney Transplant Recipients With Histological Antibody-Mediated Rejection. Front. Immunol. 2021, 12, 703457. [Google Scholar] [CrossRef] [PubMed]
- Banasik, M.; Boratynska, M.; Koscielska-Kasprzak, K.; Krajewska, M.; Mazanowska, O.; Kaminska, D.; Bartoszek, D.; Zabinska, M.; Myszka, M.; Nowakowska, B.; et al. The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes. Transpl. Immunol. 2014, 30, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philogene, M.C.; Bagnasco, S.; Kraus, E.S.; Montgomery, R.A.; Dragun, D.; Leffell, M.S.; Zachary, A.A.; Jackson, A.M. Anti-Angiotensin II Type 1 Receptor and Anti-Endothelial Cell Antibodies: A Cross-Sectional Analysis of Pathological Findings in Allograft Biopsies. Transplantation 2017, 101, 608–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banasik, M.; Boratynska, M.; Koscielska-Kasprzak, K.; Kaminska, D.; Zmonarski, S.; Mazanowska, O.; Krajewska, M.; Bartoszek, D.; Zabinska, M.; Myszka, M.; et al. Non-HLA antibodies: Angiotensin II type 1 receptor (anti-AT1R) and endothelin-1 type A receptor (anti-ETAR) are associated with renal allograft injury and graft loss. Transplant. Proc. 2014, 46, 2618–2621. [Google Scholar] [CrossRef]
- Pearl, M.H.; Chen, L.; ElChaki, R.; Elashoff, D.; Gjertson, D.W.; Rossetti, M.; Weng, P.L.; Zhang, Q.; Reed, E.F.; Chambers, E.T. Endothelin Type A Receptor Antibodies Are Associated With Angiotensin II Type 1 Receptor Antibodies, Vascular Inflammation, and Decline in Renal Function in Pediatric Kidney Transplantation. Kidney Int. Rep. 2020, 5, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, E.; Calabrese, F.; Schiavon, M.; Feltracco, P.; Seveso, M.; Carollo, C.; Loy, M.; Cardillo, M.; Rea, F. Immediate and Catastrophic Antibody-Mediated Rejection in a Lung Transplant Recipient With Anti-Angiotensin II Receptor Type 1 and Anti-Endothelin-1 Receptor Type A Antibodies. Am. J. Transplant. 2017, 17, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, U.A.; Lachmann, N.; Ranucci, G.; Sawitzki, B.; Schoenemann, C.; Pratschke, J.; Dragun, D.; Pascher, A. Non-HLA Antibodies May Accelerate Immune Responses After Intestinal and Multivisceral Transplantation. Transplantation 2017, 101, 141–149. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Demetris, A.J.; Philippe, A.; Freeman, R.; Cai, J.; Heidecke, H.; Smith, C.; Hart, B.; Jennings, L.W.; Catar, R.; et al. Non-HLA Antibodies Impact on C4d Staining, Stellate Cell Activation and Fibrosis in Liver Allografts. Transplantation 2017, 101, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Hiemann, N.E.; Meyer, R.; Wellnhofer, E.; Schoenemann, C.; Heidecke, H.; Lachmann, N.; Hetzer, R.; Dragun, D. Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation. Transplantation 2012, 94, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Reinsmoen, N.L.; Mirocha, J.; Ensor, C.R.; Marrari, M.; Chaux, G.; Levine, D.J.; Zhang, X.; Zeevi, A. A 3-Center Study Reveals New Insights Into the Impact of Non-HLA Antibodies on Lung Transplantation Outcome. Transplantation 2017, 101, 1215–1221. [Google Scholar] [CrossRef]
- Banasik, M.; Jablecki, J.; Boratynska, M.; Kaminska, D.; Koscielska-Kasprzak, K.; Bartoszek, D.; Chelmonski, A.; Halon, A.; Baran, W.; Klinger, M. Humoral immunity in hand transplantation: Anti-HLA and non-HLA response. Hum. Immunol. 2014, 75, 859–862. [Google Scholar] [CrossRef]
- Philogene, M.C.; Johnson, T.; Vaught, A.J.; Zakaria, S.; Fedarko, N. Antibodies against Angiotensin II Type 1 and Endothelin A Receptors: Relevance and pathogenicity. Hum. Immunol. 2019, 80, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, A.; Yanagisawa, M.; Kimura, S.; Kasuya, Y.; Miyauchi, T.; Goto, K.; Masaki, T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. USA 1989, 86, 2863–2867. [Google Scholar] [CrossRef] [Green Version]
- Egido, J.; Rojas-Rivera, J.; Mas, S.; Ruiz-Ortega, M.; Sanz, A.B.; Gonzalez Parra, E.; Gomez-Guerrero, C. Atrasentan for the treatment of diabetic nephropathy. Expert. Opin. Investig. Drugs 2017, 26, 741–750. [Google Scholar] [CrossRef]
- Nowańska, K.; Banasik, M.; Donizy, P.; Kościelska-Kasprzak, K.; Zmonarski, S.; Letachowicz, K.; Kamińska, D.; Mazanowska, O.; Augustyniak-Bartosik, H.; Tukiendorf, A.; et al. Endothelin A Receptors Expressed in Glomeruli of Renal Transplant Patients May Be Associated with Antibody-Mediated Rejection. J. Clin. Med. 2021, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Nowańska, K.; Donizy, P.; Kościelska-Kasprzak, K.; Kamińska, D.; Krajewska, M.; Mazanowska, O.; Madziarska, K.; Zmonarski, S.; Chudoba, P.; Małkiewicz, B.; et al. Endothelin A Receptors Expressed in Renal Blood Vessels of Renal Transplant Patients Are Connected With Acute Tubular Necrosis or Antibody-Mediated Rejection. Transplant. Proc. 2018, 50, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Banasik, M.; Boratynska, M.; Koscielska-Kasprzak, K.; Mazanowska, O.; Bartoszek, D.; Zabinska, M.; Myszka, M.; Nowakowska, B.; Halon, A.; Szyber, P.; et al. Long-term follow-up of non-HLA and anti-HLA antibodies: Incidence and importance in renal transplantation. Transplant. Proc. 2013, 45, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Tom, F. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar]
- Aziz, F.; Jung-Hynes, B.; Parajuli, S.; Redfield, R.R.; Astor, B.C.; Mandelbrot, D.; Hidalgo, L.; Djamali, A. Pre-transplant AT1R antibodies and long-term outcomes in kidney transplant recipients with a functioning graft for more than 5 years. Clin. Nephrol. 2020, 94, 245–251. [Google Scholar] [CrossRef]
- Delville, M.; Lamarthée, B.; Pagie, S.; See, S.B.; Rabant, M.; Burger, C.; Gatault, P.; Giral, M.; Thaunat, O.; Arzouk, N.; et al. Early Acute Microvascular Kidney Transplant Rejection in the Absence of Anti-HLA Antibodies Is Associated with Preformed IgG Antibodies against Diverse Glomerular Endothelial Cell Antigens. J. Am. Soc. Nephrol. 2019, 30, 692–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fichtner, A.; Süsal, C.; Höcker, B.; Rieger, S.; Waldherr, R.; Westhoff, J.H.; Sander, A.; Dragun, D.; Tönshoff, B. Association of non-HLA antibodies against endothelial targets and donor-specific HLA antibodies with antibody-mediated rejection and graft function in pediatric kidney transplant recipients. Pediatr. Nephrol. 2021, 36, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Sas, A.; Donizy, P.; Kościelska-Kasprzak, K.; Kamińska, D.; Mazanowska, O.; Krajewska, M.; Chudoba, P.; Korta, K.; Hałoń, A.; Klinger, M.; et al. Histopathological Relevance of Angiotensin II Type 1 Receptor in Renal Transplant Biopsy. Transplant. Proc. 2018, 50, 1847–1849. [Google Scholar] [CrossRef] [PubMed]
- Sas-Strózik, A.; Donizy, P.; Kościelska-Kasprzak, K.; Kamińska, D.; Gawlik, K.; Mazanowska, O.; Madziarska, K.; Hałoń, A.; Krajewska, M.; Banasik, M. Angiotensin II Type 1 Receptor Expression in Renal Transplant Biopsies and Anti-AT1R Antibodies in Serum Indicates the Risk of Transplant Loss. Transplant. Proc. 2020, 52, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Sas-Strózik, A.; Krajewska, M.; Banasik, M. The significance of angiotensin II type 1 receptor (AT1 receptor) in renal transplant injury. Adv. Clin. Exp. Med. 2020, 29, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Dragun, D.; Catar, R.; Philippe, A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney Int. 2016, 90, 280–288. [Google Scholar] [CrossRef] [PubMed]
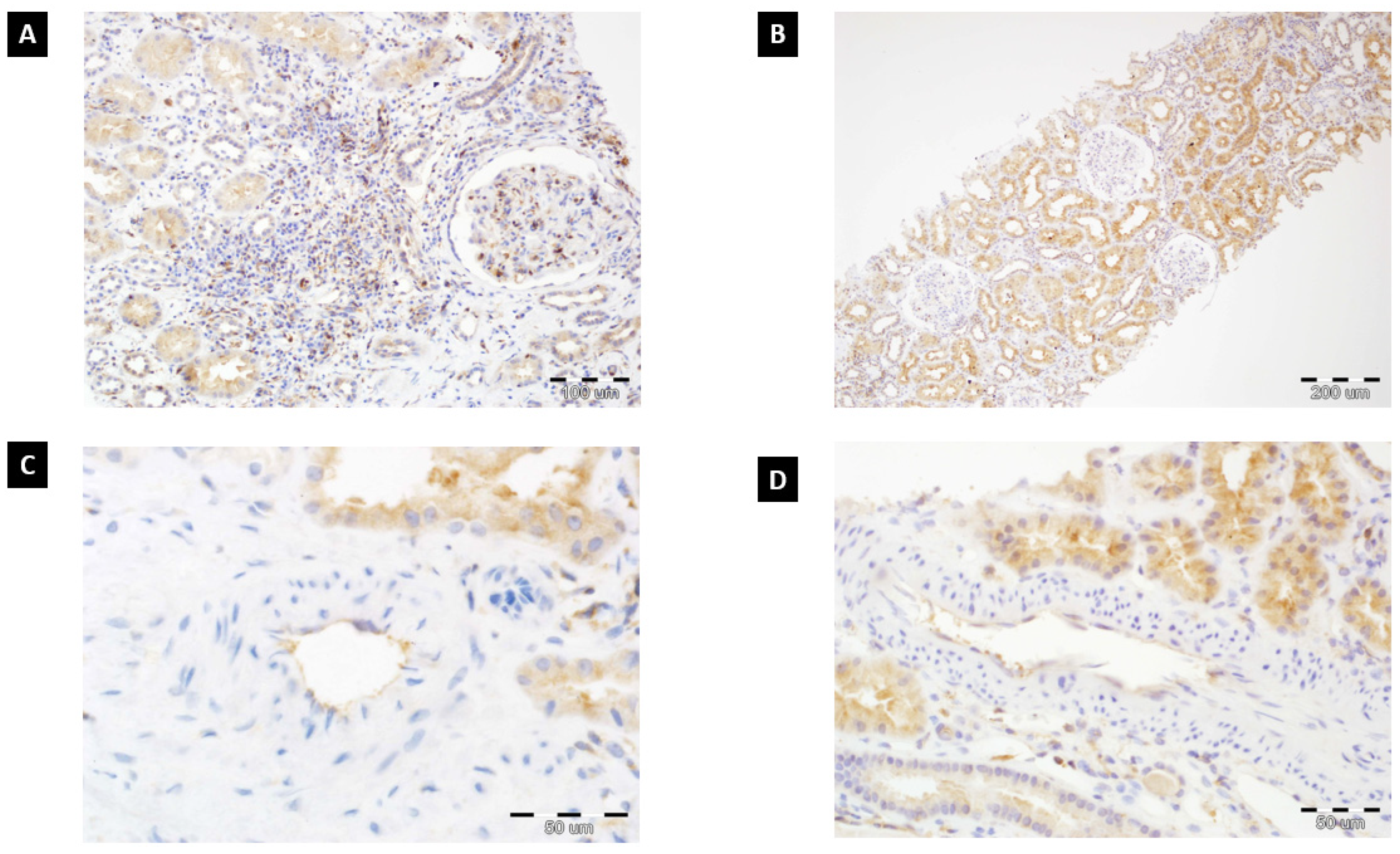

| Patient Characteristics | AMR Positive Group n = 25 | AMR Negative Group n = 131 | p |
|---|---|---|---|
| Mean summarized expression of ETA receptor score | 3.28 ± 1.56 | 1.47 ± 1.35 | <0.000001 |
| Recipient age (years) | 41.7 ± 15 | 43.4 ± 14 | NS |
| Male gender, n (%) | 16 (64%) | 88 (67.1%) | NS |
| No. of HLA ABDR mismatches | 3.75 ± 0.82 | 3.56 ± 1.1 | NS |
| A | 1.5 ± 0.5 | 1.32 ± 0.6 | NS |
| B | 1.25 ± 0.4 | 1.35 ± 0.6 | NS |
| DR | 0.9 ± 0.4 | 0.88 ± 0.6 | NS |
| No. (%) of presensitized patients | |||
| max PRA < 10% | 17 (68%) | 111 (84.7%) | 0.0001 |
| max PRA 10–50% | 7 (28%) | 13 (9.9%) | 0.01 |
| max PRA > 50% | 1 (4%) | 7 (5.3%) | NS |
| First/next transplantation | 24/1 | 121/10 | NS |
| Cold ischemia time (hours) | 22.4 ± 9.2 | 21.1 ± 9.8 | NS |
| Donor male gender (%) | 57% | 58% | NS |
| Donor age (years) | 43.7 ± 16.3 | 50.3 ± 13.2 | NS |
| Cause of chronic renal failure: | |||
| Chronic glomerulonephritis | 8 | 59 | NS |
| Diabetic nephropathy | 2 | 14 | NS |
| Hypertonic nephropathy | 3 | 17 | NS |
| Polycystic kidney disease | 3 | 12 | NS |
| Interstitial nephritis | 3 | 12 | NS |
| Other | 6 | 17 | NS |
| Initial immunosuppression | |||
| Tacrolimus | 16 (64%) | 92 (70.2%) | NS |
| Cyclosporin | 9 (36% | 39 (39.7%) | NS |
| MMF/MPA | 25 (100%) | 128 (97.7%) | NS |
| Azatioprine | 0 (0%) | 3 (2.3%) | NS |
| Steroids | 25 (100%) | 131 (100%) | NS |
| Analysis: | Univariate | Multivariate |
|---|---|---|
| Risk Factor | OR (95% CI), p-Value | OR (95% CI), p-Value |
| No. of grafts | 0.83 (0.13, 5.52), 0.8483 | 0.59 (0.06, 5.42), 0.6431 |
| Recipient age | 0.97 (0.93, 1.01), 0.1459 | 0.96 (0.91, 1.00), 0.0621 |
| Male recipient | 0.79 (0.24, 2.58), 0.6963 | 0.73 (0.20, 2.63), 0.6316 |
| Max PRA | 0.98 (0.94, 1.02), 0.3506 | 0.99 (0.95, 1.03), 0.5270 |
| No. of MM HLA ABDR | 1.21 (0.85, 1.72), 0.2991 | 1.10 (0.63, 1.93), 0.7442 |
| Anti-HLA Abs | 1.40 (0.44, 4.49), 0.5685 | 1.46 (0.41, 5.20), 0.5592 |
| Summarized ETA Receptor Expression Score | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 38 | 42 | 32 | 20 | 13 | 9 | 2 | 0 | 0 |
| Percentage of patients | 24.4 | 26.9 | 20.5 | 12.8 | 8.3 | 5.8 | 1.3 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasik, M.; Kuriata-Kordek, M.; Donizy, P.; Nowańska, K.; Wiśnicki, K.; Letachowicz, K.; Zmonarski, S.; Kamińska, D.; Mazanowska, O.; Dawiskiba, T.; et al. The Summarized Assessment of Endothelin A Receptor Expression in Renal Transplant Compartments Associated with Antibody-Mediated Rejection. Diagnostics 2021, 11, 2366. https://doi.org/10.3390/diagnostics11122366
Banasik M, Kuriata-Kordek M, Donizy P, Nowańska K, Wiśnicki K, Letachowicz K, Zmonarski S, Kamińska D, Mazanowska O, Dawiskiba T, et al. The Summarized Assessment of Endothelin A Receptor Expression in Renal Transplant Compartments Associated with Antibody-Mediated Rejection. Diagnostics. 2021; 11(12):2366. https://doi.org/10.3390/diagnostics11122366
Chicago/Turabian StyleBanasik, Mirosław, Magdalena Kuriata-Kordek, Piotr Donizy, Katarzyna Nowańska, Krzysztof Wiśnicki, Krzysztof Letachowicz, Sławomir Zmonarski, Dorota Kamińska, Oktawia Mazanowska, Tomasz Dawiskiba, and et al. 2021. "The Summarized Assessment of Endothelin A Receptor Expression in Renal Transplant Compartments Associated with Antibody-Mediated Rejection" Diagnostics 11, no. 12: 2366. https://doi.org/10.3390/diagnostics11122366
APA StyleBanasik, M., Kuriata-Kordek, M., Donizy, P., Nowańska, K., Wiśnicki, K., Letachowicz, K., Zmonarski, S., Kamińska, D., Mazanowska, O., Dawiskiba, T., Janczak, D., Hałoń, A., Kepinska, M., Uchmanowicz, B., Zachciał, J., Tukiendorf, A., & Krajewska, M. (2021). The Summarized Assessment of Endothelin A Receptor Expression in Renal Transplant Compartments Associated with Antibody-Mediated Rejection. Diagnostics, 11(12), 2366. https://doi.org/10.3390/diagnostics11122366









