Association of Corneal Biomechanics Properties with Myopia in a Child and a Parent Cohort: Hong Kong Children Eye Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Measurement of Corneal Biomechanics
2.3. Measurement of Refractive Error, Corneal Curvature and Axial Length
2.4. Statistical Analysis
3. Results
3.1. Characteristic of Children Cohort and Parent Cohort
3.2. Determinants for Corneal Biomechanics
3.3. Comparison of the Coefficient of Determinants with DA between Two Cohorts
3.4. Analysis of the Association between SE and DA
3.5. Correlation of Parental DA on Children’s DA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coppe, A.M.; Ripandelli, G.; Parisi, V.; Varano, M.; Stirpe, M. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmology 2005, 112, 2103–2109. [Google Scholar] [CrossRef]
- Fujimoto, M.; Hangai, M.; Suda, K.; Yoshimura, N. Features associated with foveal retinal detachment in myopic macular retinoschisis. Am. J. Ophthalmol. 2010, 150, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Saw, S.M.; Gazzard, G.; Shih-Yen, E.C.; Chua, W.H. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005, 25, 381–391. [Google Scholar] [CrossRef]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.-M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef]
- Lee, J.H.; Jee, D.; Kwon, J.W.; Lee, W.K. Prevalence and risk factors for myopia in a rural Korean population. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5466–5471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Du, M.; Yi, H.; Duan, S.; Guo, W.; Qin, P.; Hao, Z.; Sun, J. Prevalence of and Factors Associated with Myopia in Inner Mongolia Medical Students in China, a cross-sectional study. BMC Ophthalmol. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudnicka, A.R.; Kapetanakis, V.V.; Wathern, A.K.; Logan, N.S.; Gilmartin, B.; Whincup, P.H.; Cook, D.G.; Owen, C.G. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: Implications for aetiology and early prevention. Br. J. Ophthalmol. 2016, 100, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.J.; Shin, J.Y.; Yu, H.G. Complications of Pathologic Myopia. Eye Contact Lens 2016, 42, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, T.; Matsuura, M.; Fujino, Y.; Murata, H.; Tokumo, K.; Nakakura, S.; Kiuchi, Y.; Asaoka, R. The Relationship Between Corvis ST Tonometry Parameters and Ocular Response Analyzer Corneal Hysteresis. J. Glaucoma 2020, 29, 479–484. [Google Scholar] [CrossRef]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef]
- Qiu, K.; Lu, X.; Zhang, R.; Wang, G.; Zhang, M. Corneal Biomechanics Determination in Healthy Myopic Subjects. J. Ophthalmol. 2016, 2016, 2793516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, L.; Gazzard, G.; Chan, Y.H.; Fong, A.; Kotecha, A.; Sim, E.; Tan, D.; Tong, L.; Saw, S.-M. Cornea biomechanical characteristics and their correlates with refractive error in Singaporean children. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3852–3857. [Google Scholar] [CrossRef] [Green Version]
- Fontes, B.M.; Ambrosio, R.; Alonso, R.S., Jr.; Jardim, D.; Velarde, G.C.; Nose, W. Corneal biomechanical metrics in eyes with refraction of –19.00 to +9.00 D in healthy Brazilian patients. J. Refract. Surg. 2008, 24, 941–945. [Google Scholar]
- Hon, Y.; Lam, A.K. Corneal deformation measurement using Scheimpflug noncontact tonometry. Optom. Vis. Sci. 2013, 90, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Bak-Nielsen, S.; Pedersen, I.B.; Ivarsen, A.; Hjortdal, J. Repeatability, reproducibility, and age dependency of dynamic Scheimpflug-based pneumotonometer and its correlation with a dynamic bidirectional pneumotonometry device. Cornea 2015, 34, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, G.; Hassan, Z.; Csutak, A.; Szalai, E.; Berta, A.; Modis, L., Jr. Repeatability of ocular biomechanical data measurements with a Scheimpflug-based noncontact device on normal corneas. J. Refract. Surg. 2013, 29, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Serbecic, N.; Beutelspacher, S.; Markovic, L.; Roy, A.S.; Shetty, R. Repeatability and reproducibility of corneal biomechanical parameters derived from Corvis ST. Eur. J. Ophthalmol. 2020, 30, 1287–1294. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Jin, Y.; Yang, X.; Zhao, C.; Long, Q. Corneal Biomechanical Properties in Myopic Eyes Measured by a Dynamic Scheimpflug Analyzer. J. Ophthalmol. 2015, 2015, 161869. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, M.; Murata, H.; Fujino, Y.; Yanagisawa, M.; Nakao, Y.; Tokumo, K.; Nakakura, S.; Kiuchi, Y.; Asaoka, R. Relationship between novel intraocular pressure measurement from Corvis ST and central corneal thickness and corneal hysteresis. Br. J. Ophthalmol. 2020, 104, 563–568. [Google Scholar] [CrossRef]
- Wang, W.; He, M.; He, H.; Zhang, C.; Jin, H.; Zhong, X. Corneal biomechanical metrics of healthy Chinese adults using Corvis ST. Contact Lens Anterior Eye 2016, 40, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Maeda, N.; Ikuno, Y.; Asai, T.; Hara, C.; Nishida, K. Factors Associated with Corneal Deformation Responses Measured With a Dynamic Scheimpflug Analyzer. Investig. Ophthalmol. Vis. Sci. 2017, 58, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.; Chang, R.T.; Wong, I.Y.; Lai, J.S.; Lee, J.W.; Singh, K. Assessment of corneal biomechanical parameters in myopes and emmetropes using the Corvis ST. Clin. Exp. Optom. 2016, 99, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salouti, R.; Bagheri, M.; Shamsi, A.; Zamani, M. Corneal Parameters in Healthy Subjects Assessed by Corvis ST. J. Ophthalmic Vis. Res. 2020, 15, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Yam, J.C.; Tang, S.M.; Kam, K.W.; Chen, L.J.; Yu, M.; Law, A.K.; Yip, B.H.; Wang, Y.M.; Cheung, C.Y.L.; Ng, D.S.C.; et al. High prevalence of myopia in children and their parents in Hong Kong Chinese Population: The Hong Kong Children Eye Study. Acta Ophthalmol. 2020, 98, e639–e648. [Google Scholar] [CrossRef]
- Tang, S.M.; Kam, K.W.; French, A.N.; Yu, M.; Chen, L.J.; Young, A.L.; Rose, K.A.; Tham, C.C.; Pang, C.P.; Yam, J.C. Independent Influence of Parental Myopia on Childhood Myopia in a Dose-Related Manner in 2,055 Trios: The Hong Kong Children Eye Study. Am. J. Ophthalmol. 2020, 218, 199–207. [Google Scholar] [CrossRef]
- Chen, X.; Stojanovic, A.; Hua, Y.; Eidet, J.R.; Hu, D.; Wang, J.; Utheim, T.P. Reliability of corneal dynamic scheimpflug analyser measurements in virgin and post-PRK eyes. PLoS ONE 2014, 9, e109577. [Google Scholar]
- Hox, J.J.; Bechger, T.M. An Introduction to Structural Equation Modeling. Fam. Sci. Rev. 2007, 11, 354–373. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Narayanaswamy, A.; Chung, R.S.; Wu, R.Y.; Park, J.; Wong, W.L.; Saw, S.M.; Wang, T.Y.; Aung, T. Determinants of corneal biomechanical properties in an adult Chinese population. Ophthalmology 2011, 118, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- McBrien, N.A.; Cornell, L.M.; Gentle, A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2179–2187. [Google Scholar]
- McBrien, N.A.; Lawlor, P.; Gentle, A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3713–3719. [Google Scholar]
- McBrien, N.A.; Jobling, A.I.; Gentle, A. Biomechanics of the sclera in myopia: Extracellular and cellular factors. Optom. Vis. Sci. 2009, 86, E23–E30. [Google Scholar] [CrossRef] [PubMed]
- Gentle, A.; Liu, Y.; Martin, J.E.; Conti, G.L.; McBrien, N.A. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J. Biol. Chem. 2003, 278, 16587–16594. [Google Scholar] [CrossRef] [Green Version]
- McBrien, N.A.; Gentle, A. Role of the sclera in the development and pathological complications of myopia. Prog. Retin. Eye Res. 2003, 22, 307–338. [Google Scholar] [CrossRef]


| Total (n = 5584) | Children (n = 3643) | Adults (n = 1941) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | ||
| Deformation Amplitude (mm) | 1.04 ± 0.12 | 0.51–2.5 | 1.01 ± 0.11 | 0.51–2.5 | 1.10 ± 0.10 | 0.73–1.76 | <0.001 |
| Applanation inwards (mm) | 1.79 ± 0.14 | 0.49–3.91 | 1.81 ± 0.15 | 0.49–3.91 | 1.77 ± 0.11 | 1.21–2.79 | <0.001 |
| Velocity inwards (m/s) | 0.17 ± 0.02 | 0.02–0.8 | 0.16 ± 0.02 | 0.02–0.8 | 0.17 ± 0.02 | 0.03–0.7 | 0.003 |
| Applanation outwards (mm) | 1.61 ± 0.42 | 0.24–3.63 | 1.59 ± 0.44 | 0.24–3.63 | 1.66 ± 0.37 | 0.35–2.79 | <0.001 |
| Velocity outwards (m/s) | −0.37 ± 0.11 | −1.26–−0.01 | −0.35 ± 0.12 | −1.26–−0.01 | −0.41 ± 0.09 | −0.92–−0.09 | <0.001 |
| Peak distance (mm) | 4.18 ± 1.13 | 0.62–9.7 | 4.2 ± 1.07 | 0.62–9.7 | 4.14 ± 1.24 | 0.89–8.63 | 0.047 |
| Radius of curvature (mm) | 7.1 ± 1.53 | 0.52–13.81 | 6.95 ± 1.65 | 0.52–12.02 | 7.38 ± 1.23 | 1.53–13.81 | <0.001 |
| IOP (mmHg) | 15.45 ± 2.49 | 8–34.5 | 15.56 ± 2.59 | 8–34.5 | 15.26 ± 2.28 | 8–31 | <0.001 |
| CCT (μm) | 547.08 ± 33.05 | 404–679 | 549.86 ± 32.47 | 404–679 | 541.84 ± 33.5 | 416–661 | <0.001 |
| Corneal curvature (Diopter) | 43.6 ± 1.45 | 38.04–50.62 | 43.53 ± 1.41 | 38.04–49.27 | 43.73 ± 1.51 | 38.34–50.62 | <0.001 |
| Age (year) | 19.87 ± 16.51 | 4.41–72.49 | 7.66 ± 1.01 | 4.41–11.36 | 41.09 ± 5.93 | 24.24–72.49 | <0.001 |
| AL (mm) | 23.69 ± 1.39 | 19.55–34.94 | 23.17 ± 0.95 | 19.55–27.99 | 24.67 ± 1.55 | 21.14–34.94 | <0.001 |
| SE | −0.99 ± 2.76 | −23.5–8.25 | 0.12 ± 1.60 | −11–8.25 | −3.08 ± 3.23 | −23.5–5.88 | <0.001 |
| Number of myopia (n, %) | 2486 (44.52%) | 926 (25.41%) | 1560 (80.50%) | <0.001 | |||
| Corneal Biomechanics | Deformation Amplitude (mm) | Applanation Inwards (mm) | Velocity Inwards (m/s) | Applanation Outwards (mm) | Velocity Outwards (m/s) | Peak Distance (mm) | Radius of Curvature (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variables | ß | p value | ß | p value | ß | p value | ß | p value | ß | p value | ß | p value | |||
| Children | AL | 0.011 | <0.001 | −0.0017 | 0.647 | 7.52 × 10−4 | 0.11 | −0.0023 | 0.818 | −0.019 | <0.001 | 0.026 | 0.30 | 0.031 | 0.40 |
| IOP | −0.029 | <0.001 | 0.0070 | <0.001 | −0.0053 | <0.001 | 0.0027 | 0.389 | 0.015 | <0.001 | −0.070 | <0.001 | 0.059 | <0.001 | |
| CCT | −4.16 × 10−5 | 0.40 | 3.01 × 10−4 | <0.001 | 8.48 × 10−6 | 0.44 | 0.0019 | <0.001 | 2.67 × 10−4 | <0.001 | −5.73 × 10−4 | 0.33 | 0.0059 | <0.001 | |
| Corneal curvature | 0.0086 | <0.001 | −0.018 | <0.001 | 0.0025 | <0.001 | −0.012 | 0.060 | −0.0073 | <0.001 | −0.0005 | 0.98 | −0.13 | <0.001 | |
| Age | 0.010 | <0.001 | −0.0036 | 0.202 | −3.0 × 10−4 | 0.44 | 0.0057 | 0.470 | −0.0053 | 0.007 | −0.0017 | 0.93 | 0.092 | 1.30 × 10−3 | |
| Gender | −0.0082 | 0.011 | 0.0041 | 0.471 | 0.0015 | 0.040 | 0.032 | 0.040 | 0.0026 | 0.49 | −0.025 | 0.51 | 0.16 | 4.70 × 10−3 | |
| Adults | AL | 0.013 | <0.001 | −1.54 × 10−4 | 0.927 | 0.00089 | 0.004 | −0.022 | <0.001 | −0.013 | <0.001 | −0.004 | 0.83 | −0.078 | <0.001 |
| IOP | −0.031 | <0.001 | 0.0060 | <0.001 | −0.0051 | <0.001 | 0.013 | <0.001 | 0.018 | <0.001 | −0.043 | 0.002 | 0.11 | <0.001 | |
| CCT | −7.82 × 10−5 | 0.15 | 6.02 × 10−4 | <0.001 | 1.53 × 10−5 | 0.28 | 0.0023 | <0.001 | 4.71 × 10−4 | <0.001 | 1.48 × 10−4 | 0.87 | 6.98 × 10−3 | <0.001 | |
| Corneal curvature | 0.0096 | <0.001 | −0.011 | <0.001 | 3.07 × 10−3 | <0.001 | −0.026 | <0.001 | −6.72 × 10−3 | <0.001 | −1.93 × 10−3 | 0.92 | −0.12 | <0.001 | |
| Age | 0.0013 | <0.001 | −1.13 × 10−5 | 0.98 | −0.00039 | <0.001 | 0.0016 | 0.28 | 1.20 × 10−5 | 0.97 | 0.0037 | 0.48 | 8.13 × 10-3 | 0.093 | |
| Gender | 0.0040 | 0.28 | −6.25 × 10−4 | 0.91 | 8.86 × 10−4 | 0.36 | 0.030 | 0.10 | 0.0048 | 0.25 | −0.072 | 0.26 | 0.10 | 0.092 | |
| p value | p value | p value | p value | p value | p value | p value | |||||||||
| Two Cohorts Coefficient Comparison | AL | 0.092 | 0.031 | 0.70 | 0.26 | 0.75 | 0.13 | 0.002 | |||||||
| IOP | 0.19 | 0.77 | 0.40 | 0.073 | 0.005 | 0.064 | 0.013 | ||||||||
| CCT | 0.62 | 0.13 | 0.90 | 0.089 | 0.19 | 0.49 | 0.24 | ||||||||
| Corneal curvature | 0.29 | 0.99 | 0.25 | 0.43 | <0.001 | 0.75 | 0.037 | ||||||||
| Age | <0.001 | 0.078 | 0.94 | 0.36 | 0.001 | 0.94 | 0.002 | ||||||||
| Gender | 0.016 | 0.37 | 0.58 | 0.95 | 0.98 | 0.48 | 0.53 | ||||||||
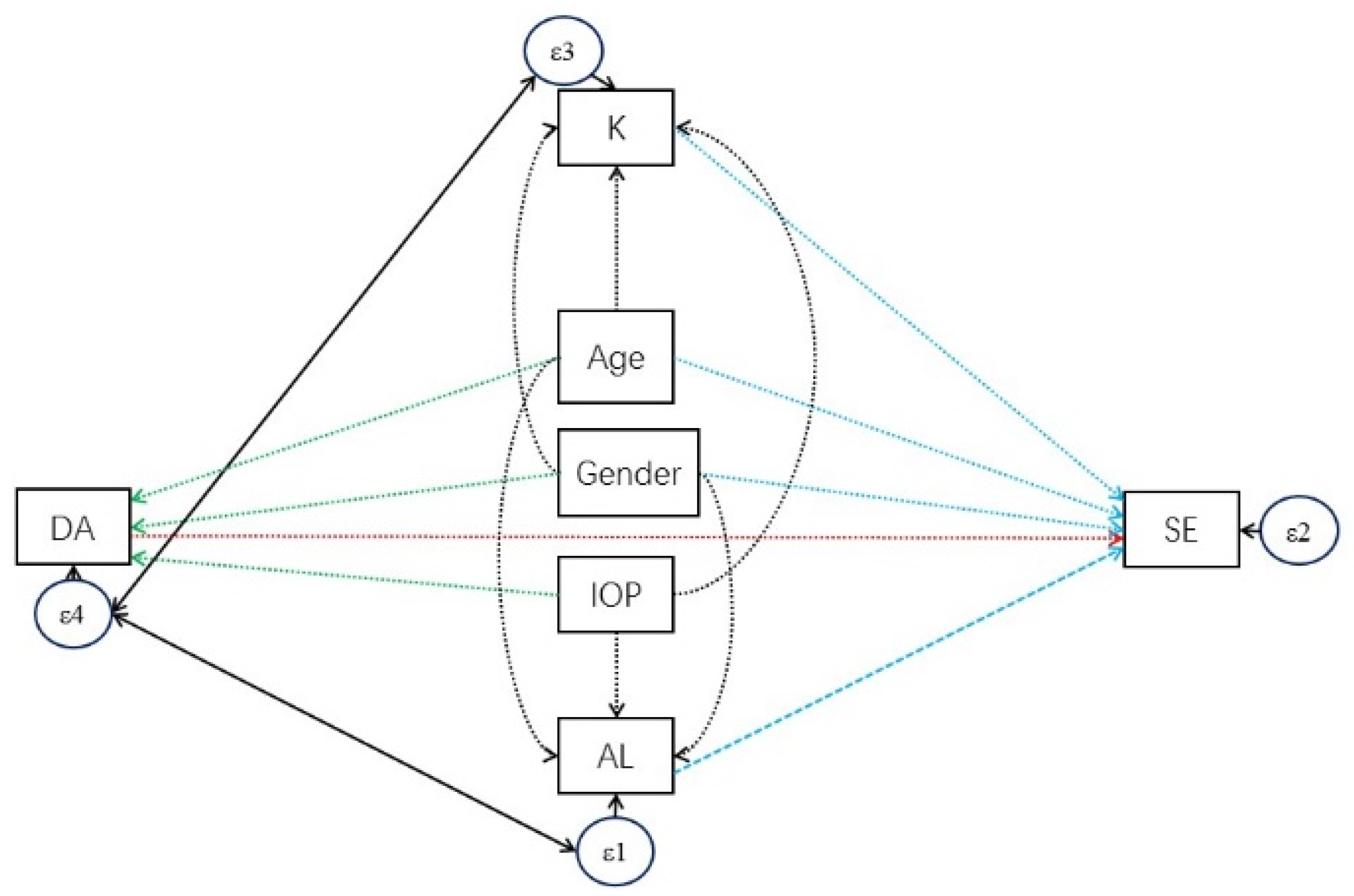
| Dependent Variables | Independent Variables | Coef. | 95% CI | p Value | |
|---|---|---|---|---|---|
| SE < -- | K_average | −0.72 | −0.7 | −0.7 | <0.001 |
| AL | −1.87 | −1.9 | −1.8 | <0.001 | |
| age | 0.13 | 0.11 | 0.16 | <0.001 | |
| gender | −0.4 | −0.5 | −0.4 | <0.001 | |
| _cons | 74.16 | 72.7 | 75.6 | <0.001 | |
| DA < -- | age | 0.01 | 0.01 | 0.02 | <0.001 |
| gender | −0.01 | −0 | 0 | 0.006 | |
| IOP | −0.03 | −0 | −0 | <0.001 | |
| _cons | 1.37 | 1.34 | 1.4 | <0.001 | |
| AL < -- | age | 0.30 | 0.27 | 0.32 | <0.001 |
| gender | −0.60 | −0.7 | −0.5 | <0.001 | |
| _cons | 21.81 | 21.6 | 22.1 | <0.001 | |
| K < -- | gender | 0.69 | 0.6 | 0.79 | <0.001 |
| _cons | 42.48 | 42.3 | 42.6 | <0.001 | |
| var(e.DA) | 0.01 | 0.01 | 0.01 | ||
| var(e.k_average) | 1.86 | 1.77 | 1.95 | ||
| var(e.SE) | 0.51 | 0.49 | 0.54 | ||
| var(e.AL) | 0.71 | 0.68 | 0.75 | ||
| cov(e.DA,e.K) | 0.02 | 0.01 | 0.02 | <0.001 | |
| cov(e.DA,e.AL) | 0.01 | 0.01 | 0.01 | <0.001 | |
| Dependent Variables | Independent Variables | Coef. | 95% CI | p Value | |
|---|---|---|---|---|---|
| SE < -- | K_average | −0.79 | −0.84 | −0.74 | 0.004 |
| AL | −1.97 | −2.02 | −1.93 | <0.001 | |
| age | 0.033 | 0.021 | 0.045 | <0.001 | |
| gender | −0.70 | −0.85 | −0.55 | <0.001 | |
| _cons | 79.91 | 77.28 | 82.54 | <0.001 | |
| DA < -- | age | 0.002 | 0.001 | 0.002 | <0.001 |
| IOP | −0.030 | −0.031 | −0.028 | <0.001 | |
| _cons | 1.49 | 1.46 | 1.52 | <0.001 | |
| AL < -- | age | 0.017 | 0.005 | 0.029 | 0.006 |
| gender | −0.57 | −0.709 | −0.423 | <0.001 | |
| IOP | 0.047 | 0.018 | 0.077 | 0.002 | |
| _cons | 24.122 | 23.358 | 24.885 | <0.001 | |
| K < -- | age | 0.018 | 0.006 | 0.030 | 0.004 |
| gender | 0.591 | 0.448 | 0.733 | <0.001 | |
| IOP | 0.068 | 0.039 | 0.097 | <0.001 | |
| _cons | 41.055 | 40.304 | 41.807 | <0.001 | |
| var(e.DA) | 0.006 | 0.006 | 0.006 | ||
| var(e.K) | 2.191 | 2.056 | 2.335 | ||
| var(e.SE) | 2.199 | 2.064 | 2.343 | ||
| var(e.AL) | 2.284 | 2.143 | 2.433 | ||
| cov(e.DA,e.K) | 0.021 | 0.016 | 0.027 | <0.001 | |
| cov(e.DA,e.AL) | 0.031 | 0.025 | 0.036 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.-M.; Zhang, X.-J.; Yu, M.; Wang, Y.-M.; Cheung, C.Y.; Kam, K.-W.; Young, A.L.; Chen, L.-J.; Tham, C.C.; Pang, C.-P.; et al. Association of Corneal Biomechanics Properties with Myopia in a Child and a Parent Cohort: Hong Kong Children Eye Study. Diagnostics 2021, 11, 2357. https://doi.org/10.3390/diagnostics11122357
Tang S-M, Zhang X-J, Yu M, Wang Y-M, Cheung CY, Kam K-W, Young AL, Chen L-J, Tham CC, Pang C-P, et al. Association of Corneal Biomechanics Properties with Myopia in a Child and a Parent Cohort: Hong Kong Children Eye Study. Diagnostics. 2021; 11(12):2357. https://doi.org/10.3390/diagnostics11122357
Chicago/Turabian StyleTang, Shu-Min, Xiu-Juan Zhang, Marco Yu, Yu-Meng Wang, Carol Y. Cheung, Ka-Wai Kam, Alvin L. Young, Li-Jia Chen, Clement C. Tham, Chi-Pui Pang, and et al. 2021. "Association of Corneal Biomechanics Properties with Myopia in a Child and a Parent Cohort: Hong Kong Children Eye Study" Diagnostics 11, no. 12: 2357. https://doi.org/10.3390/diagnostics11122357
APA StyleTang, S.-M., Zhang, X.-J., Yu, M., Wang, Y.-M., Cheung, C. Y., Kam, K.-W., Young, A. L., Chen, L.-J., Tham, C. C., Pang, C.-P., & Yam, J. C. (2021). Association of Corneal Biomechanics Properties with Myopia in a Child and a Parent Cohort: Hong Kong Children Eye Study. Diagnostics, 11(12), 2357. https://doi.org/10.3390/diagnostics11122357











