Neurogranin as a Reliable Biomarker for Synaptic Dysfunction in Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CSF Analysis
2.3. Statistical Analysis
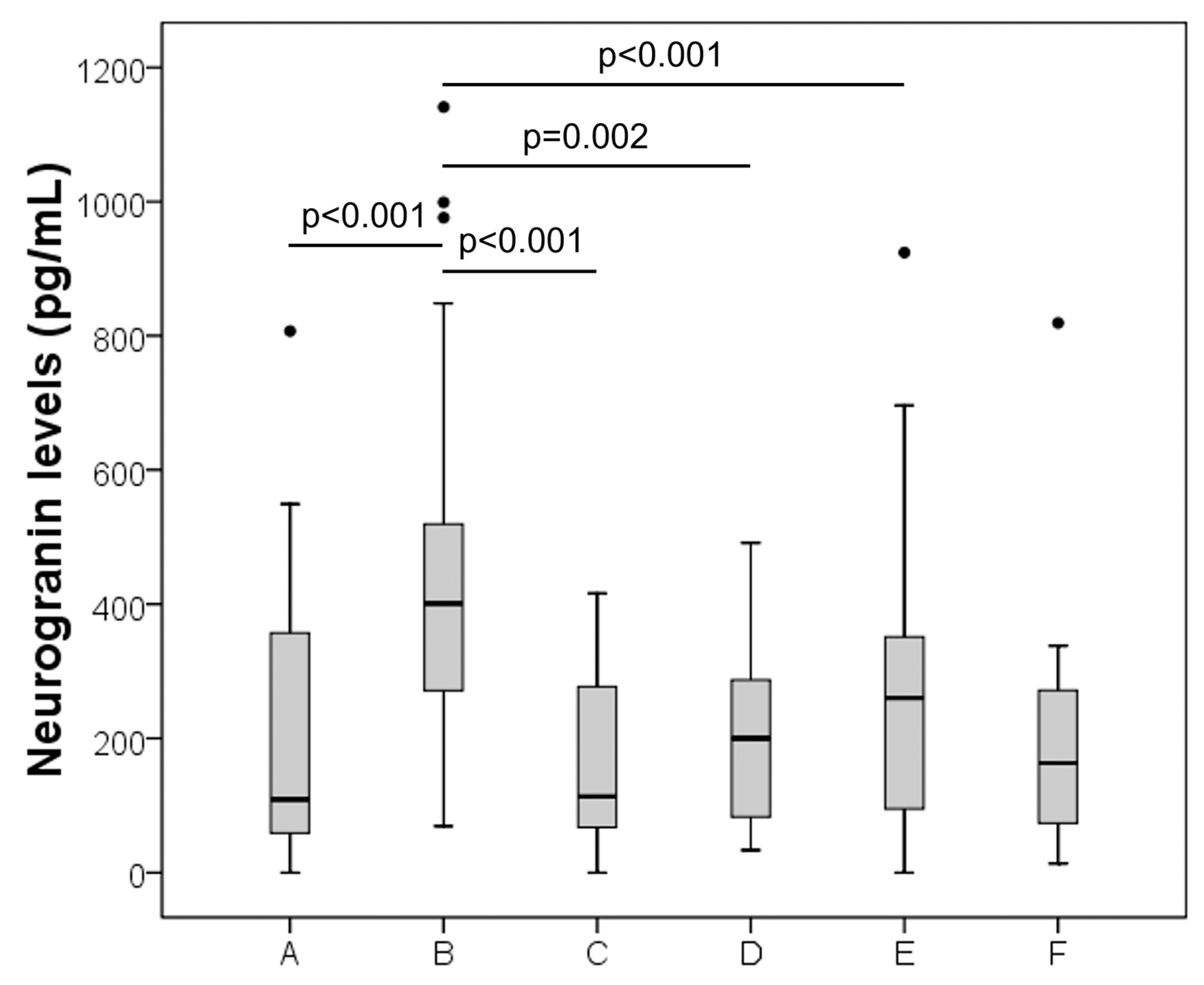
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, J.B.; Battenberg, E.F.; Wong, K.K.; Bloom, F.E.; Sutcliffe, J.G. Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein. J. Neurosci. Res. 1990, 26, 397–408. [Google Scholar] [CrossRef]
- Xiang, Y.; Xin, J.; Le, W.; Yang, Y. Neurogranin: A Potential Biomarker of Neurological and Mental Diseases. Front. Aging Neurosci. 2020, 12, 584743. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Kang, M.; Kim, Y.S.; Kim, D.H.; Nam, D.W.; Song, E.J.; Mook-Jung, I.; Moon, M. Intrahippocampal injection of a lentiviral vector expressing neurogranin enhances cognitive function in 5XFAD mice. Exp. Mol. Med. 2018, 50, e461. [Google Scholar] [CrossRef] [Green Version]
- Pak, J.H.; Huang, F.L.; Li, J.; Balschun, D.; Reymann, K.G.; Chiang, C.; Westphal, H.; Huang, K.P. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: A study with knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11232–11237. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y. Long-term potentiation: Two pathways meet at neurogranin. EMBO J. 2009, 28, 2859–2860. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Cherry, T.; Bies, C.E.; Florence, M.A.; Gerges, N.Z. Neurogranin enhances synaptic strength through its interaction with calmodulin. EMBO J. 2009, 28, 3027–3039. [Google Scholar] [CrossRef] [Green Version]
- Agnello, L.; Gambino, C.M.; Lo Sasso, B.; Bivona, G.; Milano, S.; Ciaccio, A.M.; Piccoli, T.; La Bella, V.; Ciaccio, M. Neurogranin as a Novel Biomarker in Alzheimer’s Disease. Lab. Med. 2021, 52, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Colom-Cadena, M.; Spires-Jones, T.; Zetterberg, H.; Blennow, K.; Caggiano, A.; DeKosky, S.T.; Fillit, H.; Harrison, J.E.; Schneider, L.S.; Scheltens, P.; et al. The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, E.; Beatino, M.F.; Campese, N.; Giampietri, L.; Siciliano, G.; Ceravolo, R.; Baldacci, F. Fluid Candidate Biomarkers for Alzheimer’s Disease: A Precision Medicine Approach. J. Pers. Med. 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, I. Alzheimer disease: Neurogranin in the CSF signals early Alzheimer disease and predicts disease progression. Nat. Rev. Neurol. 2015, 11, 609. [Google Scholar] [CrossRef]
- Hellwig, K.; Kvartsberg, H.; Portelius, E.; Andreasson, U.; Oberstein, T.J.; Lewczuk, P.; Blennow, K.; Kornhuber, J.; Maler, J.M.; Zetterberg, H.; et al. Neurogranin and YKL-40: Independent markers of synaptic degeneration and neuroinflammation in Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kester, M.I.; Teunissen, C.E.; Crimmins, D.L.; Herries, E.M.; Ladenson, J.H.; Scheltens, P.; van der Flier, W.M.; Morris, J.C.; Holtzman, D.M.; Fagan, A.M. Neurogranin as a Cerebrospinal Fluid Biomarker for Synaptic Loss in Symptomatic Alzheimer’s Disease. JAMA Neurol. 2015, 72, 1275–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portelius, E.; Zetterberg, H.; Skillbäck, T.; Törnqvist, U.; Andreasson, U.; Trojanowski, J.Q.; Weiner, M.W.; Shaw, L.M.; Mattsson, N.; Blennow, K.; et al. Cerebrospinal fluid neurogranin: Relation to cognition and neurodegeneration in Alzheimer’s disease. Brain 2015, 138, 3373–3385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brureau, A.; Blanchard-Bregeon, V.; Pech, C.; Hamon, S.; Chaillou, P.; Guillemot, J.-C.; Barneoud, P.; Bertrand, P.; Pradier, L.; Rooney, T.; et al. NF-L in cerebrospinal fluid and serum is a biomarker of neuronal damage in an inducible mouse model of neurodegeneration. Neurobiol. Dis. 2017, 104, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bacioglu, M.; Maia, L.F.; Preische, O.; Schelle, J.; Apel, A.; Kaeser, S.A.; Schweighauser, M.; Eninger, T.; Lambert, M.; Pilotto, A.; et al. Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron 2016, 91, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhiman, K.; Blennow, K.; Zetterberg, H.; Martins, R.N.; Gupta, V.B. Cerebrospinal fluid biomarkers for understanding multiple aspects of Alzheimer’s disease pathogenesis. Cell Mol. Life Sci. 2019, 76, 1833–1863. [Google Scholar] [CrossRef]
- Dulewicz, M.; Kulczyńska-Przybik, A.; Mroczko, B. Neurogranin and VILIP-1 as Molecular Indicators of Neurodegeneration in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 8335. [Google Scholar] [CrossRef] [PubMed]
- Rabbito, A.; Dulewicz, M.; Kulczyńska-Przybik, A.; Mroczko, B. Biochemical Markers in Alzheimer’s Disease. Int J. Mol. Sci 2020, 21, 1989. [Google Scholar] [CrossRef] [Green Version]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Del Campo, M.; Mollenhauer, B.; Bertolotto, A.; Engelborghs, S.; Hampel, H.; Simonsen, A.H.; Kapaki, E.; Kruse, N.; Le Bastard, N.; Lehmann, S.; et al. Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: An update. Biomark Med. 2012, 6, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Allegri, R.F. Moving from neurodegenerative dementias, to cognitive proteinopathies, replacing “where” by “what”…. Dement. Neuropsychol. 2020, 14, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Camporesi, E.; Nilsson, J.; Brinkmalm, A.; Becker, B.; Ashton, N.J.; Blennow, K.; Zetterberg, H. Fluid Biomarkers for Synaptic Dysfunction and Loss. Biomark. Insights 2020, 15, 1177271920950319. [Google Scholar] [CrossRef]
- Agnello, L.; Colletti, T.; Lo Sasso, B.; Vidali, M.; Spataro, R.; Gambino, C.M.; Giglio, R.V.; Piccoli, T.; Bivona, G.; La Bella, V.; et al. Tau protein as a diagnostic and prognostic biomarker in amyotrophic lateral sclerosis. Eur. J. Neurol. 2021, 28, 1868–1875. [Google Scholar] [CrossRef]
- Colletti, T.; Agnello, L.; Spataro, R.; Guccione, L.; Notaro, A.; Lo Sasso, B.; Blandino, V.; Graziano, F.; Gambino, C.M.; Giglio, R.V.; et al. Prognostic Role of CSF β-amyloid 1-42/1-40 Ratio in Patients Affected by Amyotrophic Lateral Sclerosis. Brain Sci. 2021, 11, 302. [Google Scholar] [CrossRef]
- Agnello, L.; Piccoli, T.; Vidali, M.; Cuffaro, L.; Lo Sasso, B.; Iacolino, G.; Giglio, V.R.; Lupo, F.; Alongi, P.; Bivona, G.; et al. Diagnostic accuracy of cerebrospinal fluid biomarkers measured by chemiluminescent enzyme immunoassay for Alzheimer disease diagnosis. Scand. J. Clin. Lab. Investig. 2020, 80, 313–317. [Google Scholar] [CrossRef]
- Henny, J.; Vassault, A.; Boursier, G.; Vukasovic, I.; Mesko Brguljan, P.; Lohmander, M.; Ghita, I.; Andreu, F.A.; Kroupis, C.; Sprongl, L.; et al. Recommendation for the review of biological reference intervals in medical laboratories. Clin. Chem. Lab. Med. 2016, 54, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Ciaccio, M. Vitamin D and Immunomodulation: Is It Time to Change the Reference Values? Ann. Clin. Lab. Sci. 2017, 47, 508–510. [Google Scholar] [PubMed]
- Agnello, L.; Bellia, C.; Sasso, B.L.; Pivetti, A.; Muratore, M.; Scazzone, C.; Bivona, G.; Lippi, G.; Ciaccio, M. Establishing the upper reference limit of Galectin-3 in healthy blood donors. Biochem. Med. 2017, 27, 030709. [Google Scholar] [CrossRef]
- Agnello, L.; Bellia, C.; Lo Sasso, B.; Pivetti, A.; Muratore, M.; Scazzone, C.; Bivona, G.; Lippi, G.; Ciaccio, M. Reference interval of monocyte distribution width (MDW) in healthy blood donors. Clin. Chim. Acta 2020, 510, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Bellia, C.; Scazzone, C.; Bivona, G.; Iacolino, G.; Gambino, C.M.; Muratore, M.; Lo Sasso, B.; Ciaccio, M. Establishing the 99th percentile for high sensitivity cardiac troponin I in healthy blood donors from Southern Italy. Biochem. Med. 2019, 29, 020901. [Google Scholar] [CrossRef] [PubMed]
- Ceriotti, F.; Henny, J. “Are my Laboratory Results Normal?” Considerations to be Made Concerning Reference Intervals and Decision Limits. EJIFCC 2008, 19, 106–114. [Google Scholar] [PubMed]
- Willemse, E.; Sieben, A.; Somers, C.; Vermeiren, Y.; De Roeck, N.; Timmers, M.; Van Broeckhoven, C.; De Vil, B.; Cras, P.; De Deyn, P.P.; et al. Neurogranin as biomarker in CSF is non-specific to Alzheimer’s disease dementia. Neurobiol. Aging 2021, 108, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Portelius, E.; Olsson, B.; Höglund, K.; Cullen, N.C.; Kvartsberg, H.; Andreasson, U.; Zetterberg, H.; Sandelius, Å.; Shaw, L.M.; Lee, V.; et al. Cerebrospinal fluid neurogranin concentration in neurodegeneration: Relation to clinical phenotypes and neuropathology. Acta Neuropathol. 2018, 136, 363–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Sun, F.R.; Ou, Y.N.; Shen, X.N.; Li, H.Q.; Huang, Y.Y.; Dong, Q.; Tan, L.; Yu, J.T.; Alzheimer’s Disease Neuroimaging Initiative. Association of cerebrospinal fluid neurogranin levels with cognition and neurodegeneration in Alzheimer’s disease. Aging 2020, 12, 9365–9379. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, Y.; Therriault, J.; Luo, J.; Ba, M.; Zhang, H.; Alzheimer’s Disease Neuroimaging Initiative. The Effects of CSF Neurogranin and APOE ε4 on Cognition and Neuropathology in Mild Cognitive Impairment and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 667899. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.; Xiao, M.; Xu, D.; Smirnov, D.; Salmon, D.P.; Dewit, N.; Vanbrabant, J.; Jacobs, D.; Vanderstichele, H.; Vanmechelen, E.; et al. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimer’s Dement. 2019, 5, 871–882. [Google Scholar] [CrossRef]
- Schipke, C.G.; De Vos, A.; Fuentes, M.; Jacobs, D.; Vanmechelen, E.; Peters, O. Neurogranin and BACE1 in CSF as Potential Biomarkers Differentiating Depression with Cognitive Deficits from Early Alzheimer’s Disease: A Pilot Study. Dement. Geriatr. Cogn. Disord. 2015, 8, 277–289. [Google Scholar] [CrossRef]
- Mattsson, N.; Insel, P.S.; Palmqvist, S.; Portelius, E.; Zetterberg, H.; Weiner, M.; Blennow, K.; Hansson, O.; Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol. Med. 2016, 8, 1184–1196. [Google Scholar] [CrossRef]
- Tarawneh, R.; D’Angelo, G.; Crimmins, D.; Herries, E.; Griest, T.; Fagan, A.M.; Zipfel, G.J.; Ladenson, J.H.; Morris, J.C.; Holtzman, D.M. Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease. JAMA Neurol. 2016, 73, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Hertze, J.; Zetterberg, H.; Landqvist Waldö, M.; Santillo, A.; Blennow, K.; Hansson, O. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2015, 3, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group | Sex, M | Age (Median, IQR) | Neurogranin, pg/mL (Median, IQR) |
|---|---|---|---|
| AD (n = 33) | 52% | 71 (66–77) | 401 (270–521) |
| Cerebrovascular diseases (n = 12) | 75% | 60 (52–67) | 114 (58–281) |
| Inflammatory CNS diseases (n = 10) | 30% | 64 (58–76) | 200 (76–303) |
| Peripheral Neuropathy (n = 41) | 61% | 60 (48–71) | 260 (89–352) |
| Other neurological diseases (n = 7) | 43% | 63 (48–72) | 163 (38–338) |
| Controls (n = 33) | 58% | 52 (39–67) | 109 (54–373) |
| Neurogranin | T-tau | P-tau | β42 | β40 | β42/40 Ratio | |
|---|---|---|---|---|---|---|
| Neurogranin | 0.808 p < 0.001 | 0.655 p < 0.001 | 0.053 p = 0.780 | 0.321 p = 0.118 | −0.592 p = 0.001 | |
| T-tau | 0.810 p < 0.001 | −0.030 p = 0.877 | 0.254 p = 0.221 | −0.492 p = 0.006 | ||
| P-tau | 0.013 p = 0.947 | 0.333 p = 0.104 | −0.563 p = 0.001 | |||
| β42 | 0.764 p < 0.001 | 0.463 p = 0.010 | ||||
| β40 | −0.024 p = 0.908 | |||||
| β42/40 ratio |
| Study Population | Assay | AUC | Sensitivity | Specificity | Optimal Cut-Off Value (pg/mL) | |
|---|---|---|---|---|---|---|
| Xue et al. [36] | 111 cognitively-normal controls, 193 MCI, 95 AD | Electrochemiluminescence technology using Ng7 | 0.71 | NA | NA | NA |
| Fan et al. [37] | 65 cognitively-normal controls, 65 AD | Electrochemiluminescence technology using Ng7 | 0.783 | NA | NA | NA |
| Galasko et al. [38] | 90 normal controls, 46 AD | ELISA (EUROIMMUN, Lübeck, Germany) | 0.504 | 0.200 (0.022–0.311) | 0.854 (0.640–0.932) | 167.78 |
| Schipke et al. [39] | 20 MDD, 20 AD | In-house ELISA | 0.696 | NA | NA | NA |
| Mattsson et al. [40] | 93 controls, 93 AD | In-house ELISA | 0.85 | NA | NA | 254.7 |
| Tarawneh et al. [41] | 207 controls, 95 AD | 2-site immunoassay uses an affinity-efficient trapping and purification technique for polyclonal antibodies | 0.71 | NA | NA | NA |
| Janelidze et al. [42] | 74 AD | In-house ELISA | 0.761 | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnello, L.; Lo Sasso, B.; Vidali, M.; Scazzone, C.; Piccoli, T.; Gambino, C.M.; Bivona, G.; Giglio, R.V.; Ciaccio, A.M.; La Bella, V.; et al. Neurogranin as a Reliable Biomarker for Synaptic Dysfunction in Alzheimer’s Disease. Diagnostics 2021, 11, 2339. https://doi.org/10.3390/diagnostics11122339
Agnello L, Lo Sasso B, Vidali M, Scazzone C, Piccoli T, Gambino CM, Bivona G, Giglio RV, Ciaccio AM, La Bella V, et al. Neurogranin as a Reliable Biomarker for Synaptic Dysfunction in Alzheimer’s Disease. Diagnostics. 2021; 11(12):2339. https://doi.org/10.3390/diagnostics11122339
Chicago/Turabian StyleAgnello, Luisa, Bruna Lo Sasso, Matteo Vidali, Concetta Scazzone, Tommaso Piccoli, Caterina Maria Gambino, Giulia Bivona, Rosaria Vincenza Giglio, Anna Maria Ciaccio, Vincenzo La Bella, and et al. 2021. "Neurogranin as a Reliable Biomarker for Synaptic Dysfunction in Alzheimer’s Disease" Diagnostics 11, no. 12: 2339. https://doi.org/10.3390/diagnostics11122339
APA StyleAgnello, L., Lo Sasso, B., Vidali, M., Scazzone, C., Piccoli, T., Gambino, C. M., Bivona, G., Giglio, R. V., Ciaccio, A. M., La Bella, V., & Ciaccio, M. (2021). Neurogranin as a Reliable Biomarker for Synaptic Dysfunction in Alzheimer’s Disease. Diagnostics, 11(12), 2339. https://doi.org/10.3390/diagnostics11122339











