Cytopathological Findings of Secretory Carcinoma of the Salivary Gland and the Diagnostic Utility of Giemsa Staining
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Cytological Examination
2.3. Histological Examination and Immunohistochemistry
2.4. Detection of the ETV6-NTRK3 Fusion Gene
3. Results
3.1. Clinical Findings
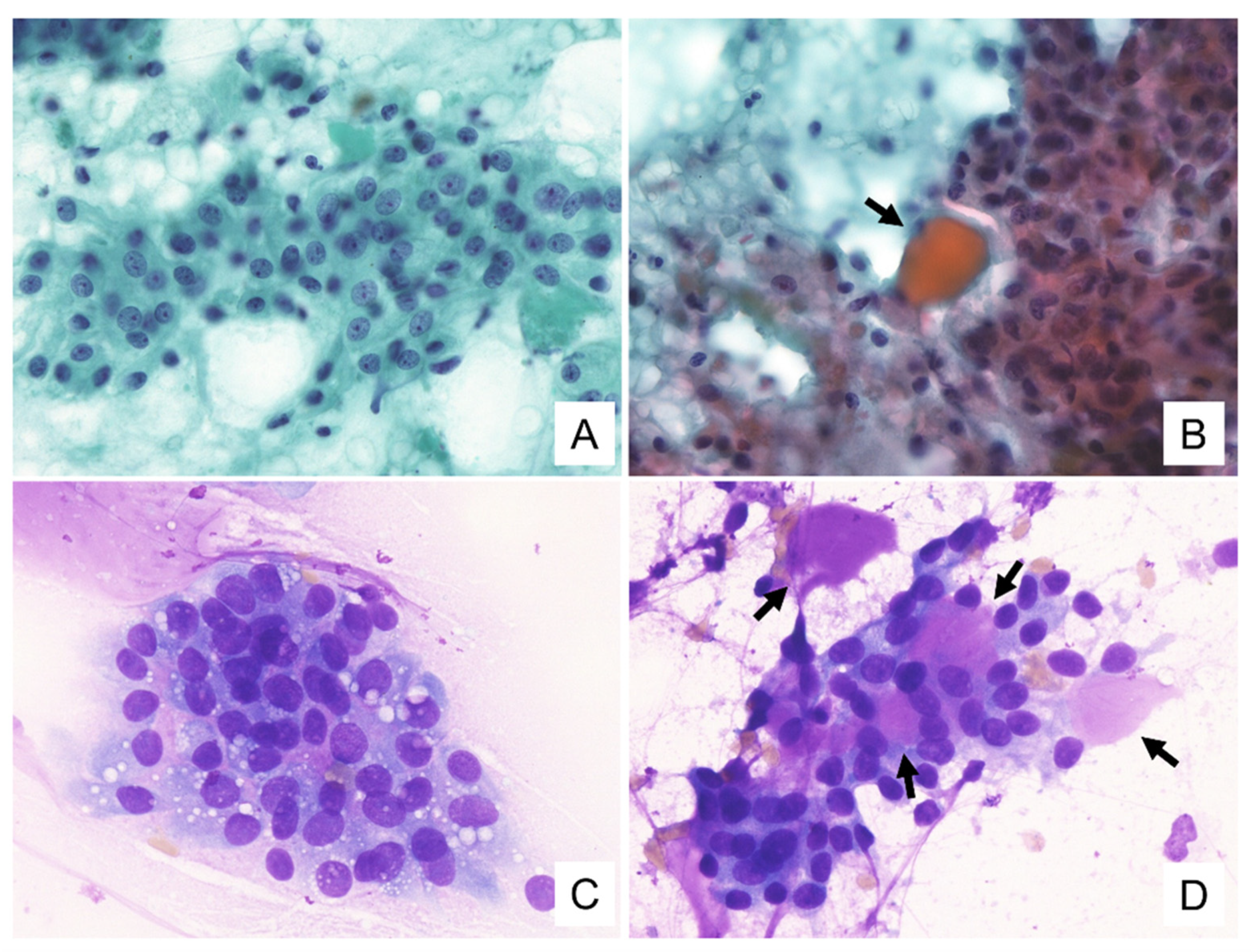
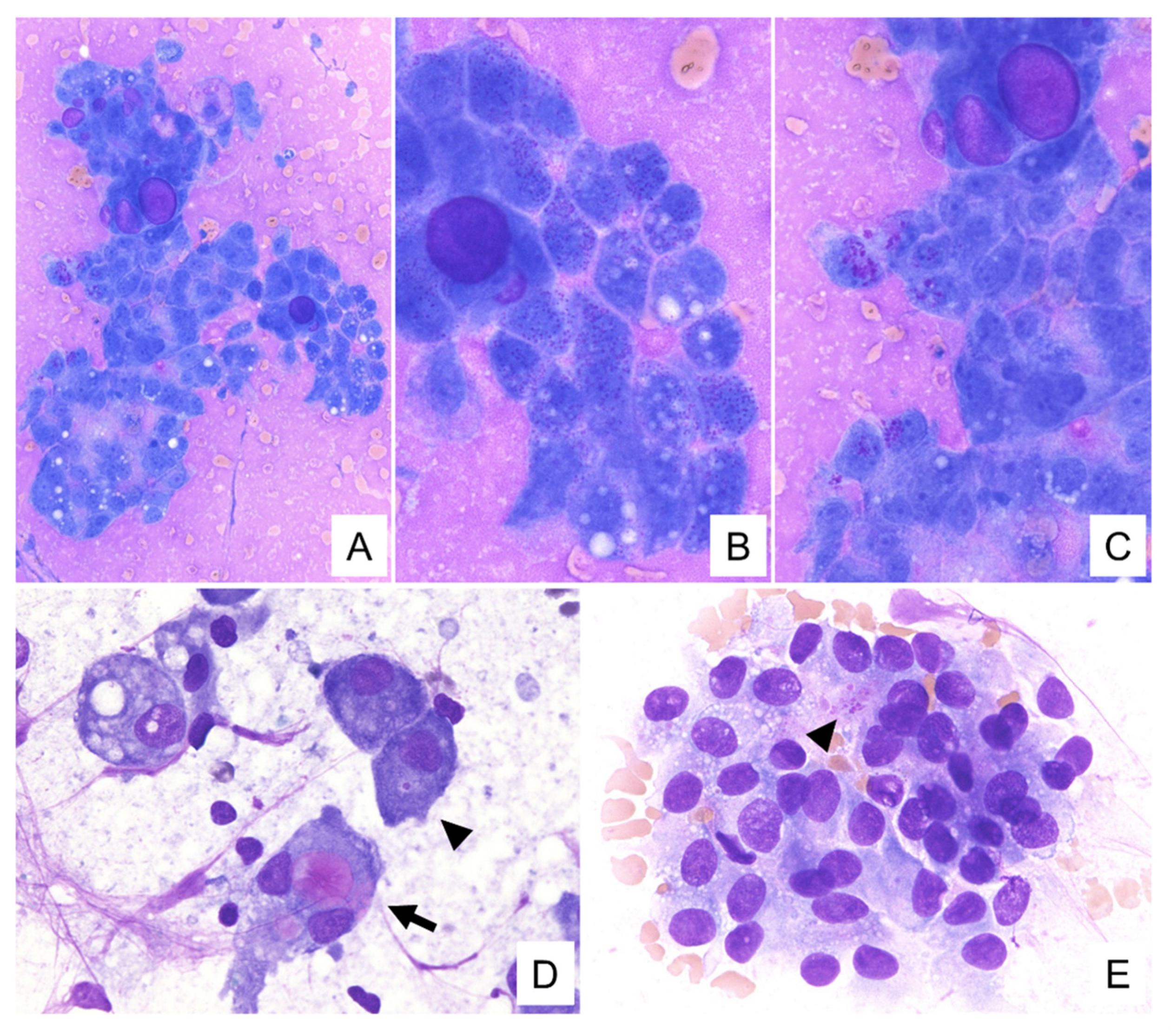
3.2. Cytological Findings
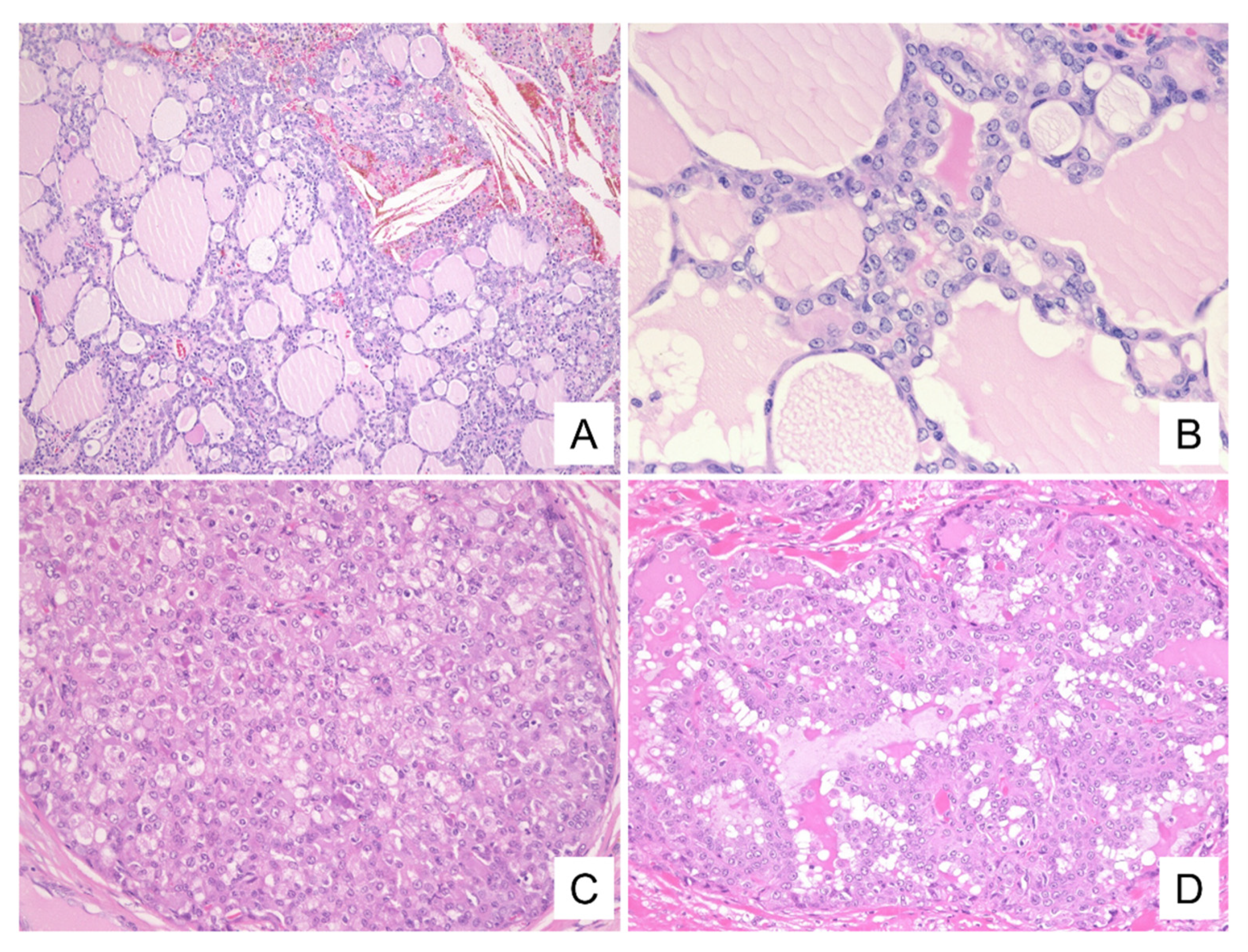
3.3. Histological and Immunohistochemical Findings
3.4. ETV6-NTRK3 Fusion Gene
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skálová, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.; Passador-Santos, F.; et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Classification of Head and Neck Tumors, 4th ed.; WHO: Geneva, Switzerland, 2017; Volume 9, pp. 177–178. [Google Scholar]
- Chiosea, S.I.; Griffith, C.; Assaad, A.; Seethala, R.R. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology 2012, 61, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Nasu, A.; Hata, S.; Fjita, M.; Yamauchi, T.; Nakamura, S.; Tanaka, K.; Ichimura, K.; Yanai, H. Mammary analogue secretory carcinoma of parotid gland—A case report. J. Jpn. Soc. Clin. Cytol. 2016, 55, 112–116. (In Japanese) [Google Scholar] [CrossRef]
- Higuchi, K.; Urano, M.; Takahashi, R.H.; Oshiro, H.; Matsubayashi, J.; Nagai, T.; Obikane, H.; Shimojo, H.; Nagao, T. Cytological features of mammary analogue secretory carcinoma of salivary gland: Fine-needle aspiration of seven cases. Diagn. Cytopathol. 2014, 42, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A.; Yonescu, R.; Batista, D.; Eisele, D.W.; Westra, W.H. Most nonparotid “acinic cell carcinomas” represent mammary analog secretory carcinomas. Am. J. Surg. Pathol. 2013, 37, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Levine, P.; Fried, K.; Krevitt, L.D.; Wang, B.; Wenig, B.M. Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: Another diagnostic dilemma in matrix-containing tumors of the salivary glands. Diagn. Cytopathol. 2014, 42, 49–53. [Google Scholar] [CrossRef]
- Khalele, B.A. Systematic review of mammary analog secretory carcinoma of salivary glands at 7 years after description. Head Neck 2017, 39, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Song, J.S.; Kim, S.Y.; Nam, S.Y.; Roh, J.L.; Choi, S.H.; Kim, S.B.; Cho, K.J. Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Korean J. Pathol. 2013, 47, 36–43. [Google Scholar] [CrossRef]
- Jung, M.J.; Kim, S.Y.; Nam, S.Y.; Roh, J.L.; Choi, S.H.; Lee, J.H.; Baek, J.H.; Cho, K.J. Aspiration cytology of mammary analogue secretory carcinoma of the salivary gland. Diagn. Cytopathol. 2015, 43, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sethi, R.; Kozin, E.; Remenschneider, A.; Meier, J.; VanderLaan, P.; Faquin, W.; Deschler, D.; Frankenthaler, R. Mammary analogue secretory carcinoma: Update on a new diagnosis of salivary gland malignancy. Laryngoscope 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Skálová, A.; Vanecek, T.; Majewska, H.; Laco, J.; Grossmann, P.; Simpson, R.H.; Hauer, L.; Andrle, P.; Hosticka, L.; Branžovský, J.; et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: Report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, β-catenin, EGFR, and CCND1 genes. Am. J. Surg. Pathol. 2014, 38, 23–33. [Google Scholar] [CrossRef]
- Pisharodi, L. Mammary analog secretory carcinoma of salivary gland: Cytologic diagnosis and differential diagnosis of an unreported entity. Diagn. Cytopathol. 2013, 41, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A.; Yonescu, R.; Batista, D.A.; Westra, W.H.; Ali, S.Z. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol. 2013, 121, 228–233. [Google Scholar] [CrossRef]
- Petersson, F.; Lian, D.; Chau, Y.P.; Yan, B. Mammary analogue secretory carcinoma: The first submandibular case reported including findings on fine needle aspiration cytology. Head Neck Pathol. 2012, 6, 135–139. [Google Scholar] [CrossRef][Green Version]
- Griffith, C.C.; Stelow, E.B.; Saqi, A.; Khalbuss, W.E.; Schneider, F.; Chiosea, S.I.; Seethala, R.R. The cytological features of mammary analogue secretory carcinoma: A series of 6 molecularly confirmed cases. Cancer Cytopathol. 2013, 121, 234–241. [Google Scholar] [CrossRef]
- Takeda, M.; Kasai, T.; Morita, K.; Takeuchi, M.; Nishikawa, T.; Yamashita, A.; Mikami, S.; Hosoi, H.; Ohbayashi, C. Cytopathological features of mammary analogue secretory carcinoma—Review of literature. Diagn. Cytopathol. 2015, 43, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Oza, N.; Sanghvi, K.; Shet, T.; Patil, A.; Menon, S.; Ramadwar, M.; Kane, S. Mammary analogue secretory carcinoma of parotid: Is preoperative cytological diagnosis possible? Diagn. Cytopathol. 2016, 44, 519–525. [Google Scholar] [CrossRef]
- Rodríguez-Urrego, P.A.; Dogan, S.; Lin, O. Cytologic findings of mammary analogue secretory carcinoma arising in the thyroid. Diagn. Cytopathol. 2017, 45, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Dhiman, A.; Gray, J.A. Fine Needle Aspiration Cytology for Neck Masses in Childhood. An Illustrative Approach. Diagnostics 2018, 8, 28. [Google Scholar] [CrossRef]
- Jain, M.; Majumdar, D.D.; Agarwal, K.; Bais, A.S.; Choudhury, M. FNAC as a diagnostic tool in pediatric head and neck lesions. Indian Pediatr. 1999, 36, 921–923. [Google Scholar]
- Mittra, P.; Bharti, R.; Pandey, M.K. Role of fine needle aspiration cytology in head and neck lesions of paediatric age group. J. Clin. Diagn. Res. 2013, 7, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Rapkiewicz, A.; Thuy Le, B.; Simsir, A.; Cangiarella, J.; Levine, P. Spectrum of head and neck lesions diagnosed by fine-needle aspiration cytology in the pediatric population. Cancer 2007, 111, 242–251. [Google Scholar] [CrossRef]
- Handa, U.; Mohan, H.; Bal, A. Role of fine needle aspiration cytology in evaluation of paediatric lymphadenopathy. Cytopathology 2003, 14, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Solomon, I.H.; El-Mofty, S.K.; Lewis, J.S., Jr.; Chernock, R.D. Mammaglobin and S-100 immunoreactivity in salivary gland carcinomas other than mammary analogue secretory carcinoma. Hum. Pathol. 2013, 44, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tognon, C.E.; Godinho, F.J.; Yasaitis, L.; Hock, H.; Herschkowitz, J.I.; Lannon, C.L.; Cho, E.; Kim, S.J.; Bronson, R.T.; et al. ETV6-NTRK3 fusion oncogene initiates breast cancer from committed mammary progenitors via activation of AP1 complex. Cancer Cell 2007, 12, 542–558. [Google Scholar] [CrossRef]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef]
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 1998, 18, 184–187. [Google Scholar] [CrossRef]
- Knezevich, S.R.; Garnett, M.J.; Pysher, T.J.; Beckwith, J.B.; Grundy, P.E.; Sorensen, P.H. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998, 58, 5046–5048. [Google Scholar] [PubMed]
- Rubin, B.P.; Chen, C.J.; Morgan, T.W.; Xiao, S.; Grier, H.E.; Kozakewich, H.P.; Perez-Atayde, A.R.; Fletcher, J.A. Congenital mesoblastic nephroma t(12; 15) is associated with ETV6-NTRK3 gene fusion: Cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am. J. Pathol. 1998, 153, 1451–1458. [Google Scholar] [CrossRef]
- Eguchi, M.; Eguchi-Ishimae, M.; Tojo, A.; Morishita, K.; Suzuki, K.; Sato, Y.; Kudoh, S.; Tanaka, K.; Setoyama, M.; Nagamura, F.; et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12; 15)(p13; q25). Blood 1999, 93, 1355–1363. [Google Scholar] [CrossRef]
- Haimoto, H.; Hosoda, K.; Kato, K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab. Investig. 1987, 57, 489–498. [Google Scholar] [PubMed]


| Case No. | Age/Sex | Location | Size (cm) | TNM | Stage | Original FNAC Diagnosis | Follow-Up |
|---|---|---|---|---|---|---|---|
| 1 | 39/F | Left parotid gland | 1.8 | cT1N0M0 pT1 | cStage I pStage I | Indeterminate/neoplastic lesion | NED, 11 years |
| 2 | 61/M | Left parotid gland | 3.0 | cT2N0M0 pT3N0 | cStage II pStage III | Carcinoma | DOD, 7 years |
| 3 | 47/M | Right parotid gland | 1.5 | cT1N0M0 pT1 | cStage I pStage I | MASC | NED, 3 years |
| 4 | 74/M | Right parotid gland | 2.8 | cT4aN0M0 pT4a | cStage IVA pStage IVA | Pleomorphic adenoma | NED, 9 months |
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Histologic findings | Follicular/Microcystic/Solid/partially Papillary | Follicular/Microcystic/Solid | Solid/Microcystic/partially Papillary | Follicular/Papillary/Solid |
| Cytologic findings | ||||
| Cellularity | High | High | High | High |
| Cluster patterns | Papillary and dendritic structures with transgressing vessels/tubular gland structure with metachromatic hyaline globules | Papillary and dendritic clusters with transgressing vessels/tubular gland structure with metachromatic hyaline globules | Papillary clusters/tubular gland structure | Papillary clusters with transgressing vessels/tubular gland structure with metachromatic hyaline globules |
| Nuclei features | Round to oval; fine chromatin; prominent nucleoli | Round to oval; fine chromatin; prominent nucleoli | Round to oval; fine chromatin; prominent nucleoli | Round to oval; fine chromatin; prominent nucleoli |
| Papanicolaou staining background | Hemorrhagic, mucinous and cystic | Cystic | Mucinous and cystic | Mucinous and cystic |
| Cytoplasmic features | Variously-sized vacuoles, occasionally with ICL | Variously-sized vacuoles occasionally with ICL | Variously-sized vacuoles | Indistinct vacuoles |
| Giemsa staining background | Metachromatic mucin | Metachromatic mucin | Metachromatic mucin | Metachromatic mucin |
| Cytoplasmic features | Variously-sized vacuoles, metachromatic secretions and granules | Variously-sized vacuoles, metachromatic secretions and granules | Variously-sized vacuoles, metachromatic secretions and granules | Variously-sized vacuoles, metachromatic secretions and granules |
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| S-100 | + | + | + | + |
| mammaglobin | + | + | + | partially + |
| GCDFP-15 | +(Secretion) | +(Secretion) | ND | partially + |
| CAM5.2 | + | ND | ND | ND |
| EMA | partially + | ND | ND | ND |
| p63 | - | ND | ND | ND |
| α-SMA | - | ND | ND | ND |
| GFAP | - | ND | ND | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egusa, Y.; Nishimura, M.F.; Baba, S.; Takeuchi, K.; Makino, T.; Tachibana, T.; Nishikori, A.; Fujita, A.; Yanai, H.; Sato, Y. Cytopathological Findings of Secretory Carcinoma of the Salivary Gland and the Diagnostic Utility of Giemsa Staining. Diagnostics 2021, 11, 2284. https://doi.org/10.3390/diagnostics11122284
Egusa Y, Nishimura MF, Baba S, Takeuchi K, Makino T, Tachibana T, Nishikori A, Fujita A, Yanai H, Sato Y. Cytopathological Findings of Secretory Carcinoma of the Salivary Gland and the Diagnostic Utility of Giemsa Staining. Diagnostics. 2021; 11(12):2284. https://doi.org/10.3390/diagnostics11122284
Chicago/Turabian StyleEgusa, Yuria, Midori Filiz Nishimura, Satoko Baba, Kengo Takeuchi, Takuma Makino, Tomoyasu Tachibana, Asami Nishikori, Azusa Fujita, Hiroyuki Yanai, and Yasuharu Sato. 2021. "Cytopathological Findings of Secretory Carcinoma of the Salivary Gland and the Diagnostic Utility of Giemsa Staining" Diagnostics 11, no. 12: 2284. https://doi.org/10.3390/diagnostics11122284
APA StyleEgusa, Y., Nishimura, M. F., Baba, S., Takeuchi, K., Makino, T., Tachibana, T., Nishikori, A., Fujita, A., Yanai, H., & Sato, Y. (2021). Cytopathological Findings of Secretory Carcinoma of the Salivary Gland and the Diagnostic Utility of Giemsa Staining. Diagnostics, 11(12), 2284. https://doi.org/10.3390/diagnostics11122284






