Fully Automated Quantitative Measurement of Serum Organic Acids via LC-MS/MS for the Diagnosis of Organic Acidemias: Establishment of an Automation System and a Proof-of-Concept Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Optimizing Multiple Reaction Mode (MRM) Transitions of 3-NPH-Derivatized Organic Acids
2.3. Sample Preparation
2.4. LC-MS/MS
2.5. Organic Acid Extraction Conditions for Serum Samples
2.6. Optimization of 3-NPH Derivatization Condition in Serum
2.7. Method Validation
2.8. Proof-of-Concept Validation
3. Results
3.1. Optimization for MS Detection Parameters for 3-NPH Derivatized Organic Acids
3.2. Optimization of LC Separation Conditions
3.3. Extraction Conditions for Serum Organic Acids
3.4. Optimization of the 3-NPH Derivatization Reaction
3.5. Linearity, Sensitivity, Accuracy, and Imprecision
3.6. Proof-of-Concept Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Millington, D.S.; Kodo, N.; Norwood, D.L.; Roe, C.R. Tandem Mass Spectrometry: A New Method for Acylcarnitine Profiling with Potential for Neonatal Screening for Inborn Errors of Metabolism. J. Inherit. Metab. Dis. 1990, 13, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.S.; Ozand, P.T.; Bucknall, M.P.; Little, D. Diagnosis of Inborn Errors of Metabolism from Blood Spots by Acylcarnitines and Amino Acids Profiling Using Automated Electrospray Tandem Mass Spectrometry. Pediatr. Res. 1995, 38, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, A.; Lindner, M.; Kohlmüller, D.; Olgemöller, K.; Mayatepek, E.; Hoffmann, G.F. Expanded Newborn Screening for Inborn Errors of Metabolism by Electrospray Ionization-Tandem Mass Spectrometry: Results, Outcome, and Implications. Pediatrics 2003, 111, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; West-Dull, A.; Hine, D.G.; Lynn, T.B.; Lowe, T. Gas-Chromatographic Method of Analysis for Urinary Organic Acids. II. Description of the Procedure, and Its Application to Diagnosis of Patients with Organic Acidurias. Clin. Chem. 1980, 26, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Kuhara, T. A New Chemical Diagnostic Method for Inborn Errors of Metabolism by Mass Spectrometry—Rapid, Practical, and Simultaneous Urinary Metabolites Analysis. Mass Spectrom. Rev. 1996, 15, 43–57. [Google Scholar] [CrossRef]
- Ohie, T.; Fu, X.-W.; Iga, M.; Kimura, M.; Yamaguchi, S. Gas Chromatography-Mass Spectrometry with Tert.-Butyldimethylsilyl Derivatization: Use of the Simplified Sample Preparations and the Automated Data System to Screen for Organic Acidemias. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 63–73. [Google Scholar] [CrossRef]
- Deodato, F.; Boenzi, S.; Santorelli, F.M.; Dionisi-Vici, C. Methylmalonic and Propionic Aciduria. Am. J. Med. Genet.-Semin. Med. Genet. 2006, 142C, 104–112. [Google Scholar] [CrossRef]
- Bannwart, C.; Wermuth, B.; Baumgartner, R.; Suormala, T.; Wiesmann, U.N. Isolated Biotin-Resistant Deficiency of 3-Methylcrotonyl-CoA Carboxylase Presenting as a Clinically Severe Form in a Newborn with Fatal Outcome. J. Inherit. Metab. Dis. 1992, 15, 863–868. [Google Scholar] [CrossRef]
- Vockley, J.; Ensenauer, R. Isovaleric Acidemia: New Aspects of Genetic and Phenotypic Heterogeneity. Am. J. Med. Genet.-Semin. Med. Genet. 2006, 142C, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Seger, C.; Shipkova, M.; Christians, U.; Billaud, E.M.; Wang, P.; Holt, D.W.; Brunet, M.; Kunicki, P.K.; Pawinski, T.; Langman, L.J.; et al. Assuring the Proper Analytical Performance of Measurement Procedures for Immunosuppressive Drug Concentrations in Clinical Practice: Recommendations of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology Immunosuppressiv. Ther. Drug Monit. 2016, 38, 170–189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, R. Recent Advances in Analytical Methods for the Therapeutic Drug Monitoring of Immunosuppressive Drugs. Drug Test. Anal. 2018, 10, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Keevil, B.G. LC–MS/MS Analysis of Steroids in the Clinical Laboratory. Clin. Biochem. 2016, 49, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Plenis, A.; Olędzka, I.; Kowalski, P.; Miękus, N.; Bączek, T. Recent Trends in the Quantification of Biogenic Amines in Biofluids as Biomarkers of Various Disorders: A Review. J. Clin. Med. 2019, 8, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, S.S.-C.; Bedner, M.; Phinney, K.W. Development of a Candidate Reference Measurement Procedure for the Determination of 25-Hydroxyvitamin D₃ and 25-Hydroxyvitamin D₂ in Human Serum Using Isotope-Dilution Liquid Chromatography Tandem Mass Spectrometry. Anal. Chem. 2010, 82, 1942–1948. [Google Scholar] [CrossRef] [Green Version]
- Stepman, H.C.M.; Vanderroost, A.; Van Uytfanghe, K.; Thienpont, L.M. Candidate Reference Measurement Procedures for Serum 25-Hydroxyvitamin D₃ and 25-Hydroxyvitamin D₂ by Using Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry. Clin. Chem. 2011, 57, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, M.R.; Hörster, F.; Dionisi-Vici, C.; Haliloglu, G.; Karall, D.; Chapman, K.A.; Huemer, M.; Hochuli, M.; Assoun, M.; Ballhausen, D.; et al. Proposed Guidelines for the Diagnosis and Management of Methylmalonic and Propionic Acidemia. Orphanet J. Rare Dis. 2014, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Fraser, J.L.; Venditti, C.P. Methylmalonic and Propionic Acidemias: Clinical Management Update. Curr. Opin. Pediatr. 2016, 28, 682–693. [Google Scholar] [CrossRef]
- Grünert, S.C.; Stucki, M.; Morscher, R.J.; Suormala, T.; Bürer, C.; Burda, P.; Christensen, E.; Ficicioglu, C.; Herwig, J.; Kölker, S.; et al. 3-Methylcrotonyl-CoA Carboxylase Deficiency: Clinical, Biochemical, Enzymatic and Molecular Studies in 88 Individuals. Orphanet J. Rare Dis. 2012, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Grebe, S.K.G.; Singh, R.J. LC-MS/MS in the Clinical Laboratory—Where to from Here? Clin. Biochem. Rev. 2011, 32, 5–31. [Google Scholar]
- Vogeser, M.; Kirchhoff, F. Progress in Automation of LC-MS in Laboratory Medicine. Clin. Biochem. 2011, 44, 4–13. [Google Scholar] [CrossRef]
- Zhang, Y.V.; Rockwood, A. Impact of Automation on Mass Spectrometry. Clin. Chim. Acta 2015, 450, 298–303. [Google Scholar] [CrossRef]
- Robin, T.; Barnes, A.; Dulaurent, S.; Loftus, N.; Baumgarten, S.; Moreau, S.; Marquet, P.; El Balkhi, S.; Saint-Marcoux, F. Fully Automated Sample Preparation Procedure to Measure Drugs of Abuse in Plasma by Liquid Chromatography Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2018, 410, 5071–5083. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0-The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- A, J.; Trygg, J.; Gullberg, J.; Johansson, A.I.; Jonsson, P.; Antti, H.; Marklund, S.L.; Moritz, T. Extraction and GC/MS Analysis of the Human Blood Plasma Metabolome. Anal. Chem. 2005, 77, 8086–8094. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-Dependent Metabolite Distribution, Clustering, and Protein Extraction for Serum Profiling with Mass Spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Minero, C.; Vincenti, M.; Lago, S.; Pelizzetti, E. Determination of Trace Amounts of Highly Hydrophilic Compounds in Water by Direct Derivatization and Gas Chromatography—Mass Spectrometry. Fresenius J. Anal. Chem. 1994, 350, 403–409. [Google Scholar] [CrossRef]
- Han, J.; Gagnon, S.; Eckle, T.; Borchers, C.H. Metabolomic Analysis of Key Central Carbon Metabolism Carboxylic Acids as Their 3-Nitrophenylhydrazones by UPLC/ESI-MS. Electrophoresis 2013, 34, 2891–2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An Isotope-Labeled Chemical Derivatization Method for the Quantitation of Short-Chain Fatty Acids in Human Feces by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Han, J.; Higgins, R.; Lim, M.D.; Atkinson, K.; Yang, J.; Lin, K.; Borchers, C.H. Isotope-Labeling Derivatization with 3-Nitrophenylhydrazine for LC/Multiple-Reaction Monitoring-Mass-Spectrometry-Based Quantitation of Carnitines in Dried Blood Spots. Anal. Chim. Acta 2018, 1037, 177–187. [Google Scholar] [CrossRef]
- Joo, K.-M.; Choi, D.; Park, Y.-H.; Yi, C.-G.; Jeong, H.-J.; Cho, J.-C.; Lim, K.-M. A Rapid and Highly Sensitive UPLC-MS/MS Method Using Pre-Column Derivatization with 2-Picolylamine for Intravenous and Percutaneous Pharmacokinetics of Valproic Acid in Rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 938, 35–42. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Ji, H.; Wang, Y.; Zhang, Z.; Lu, H. Enhancing Coverage in LC–MS-Based Untargeted Metabolomics by a New Sample Preparation Procedure Using Mixed-Mode Solid-Phase Extraction and Two Derivatizations. Anal. Bioanal. Chem. 2019, 411, 6189–6202. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.-L.; Liu, P.; Wang, Q.-Y.; Cai, W.-J.; Yuan, B.-F.; Feng, Y.-Q. Derivatization for Liquid Chromatography-Mass Spectrometry. TrAC-Trends Anal. Chem. 2014, 59, 121–132. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Clarke, N.J. Advantages and Challenges of Mass Spectrometry Assays for Steroid Hormones. J. Steroid Biochem. Mol. Biol. 2010, 121, 491–495. [Google Scholar] [CrossRef]
- Honda, A.; Yamashita, K.; Miyazaki, H.; Shirai, M.; Ikegami, T.; Xu, G.; Numazawa, M.; Hara, T.; Matsuzaki, Y. Highly Sensitive Analysis of Sterol Profiles in Human Serum by LC-ESI-MS/MS. J. Lipid Res. 2008, 49, 2063–2073. [Google Scholar] [CrossRef] [Green Version]
- Honda, A.; Miyazaki, T.; Ikegami, T.; Iwamoto, J.; Yamashita, K.; Numazawa, M.; Matsuzaki, Y. Highly Sensitive and Specific Analysis of Sterol Profiles in Biological Samples by HPLC-ESI-MS/MS. J. Steroid Biochem. Mol. Biol. 2010, 121, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T. Gas Chromatographic-Mass Spectrometric Urinary Metabolome Analysis to Study Mutations of Inborn Errors of Metabolism. Mass Spectrom. Rev. 2005, 24, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Fitzpatrick, P.; Tosif, S.; Hopper, S.M.; Donath, S.M.; Bryant, P.A.; Babl, F.E. Faster Clean Catch Urine Collection (Quick-Wee Method) from Infants: Randomised Controlled Trial. BMJ 2017, 357, j1341. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, J.; Tosif, S.; Fitzpatrick, P.; Hopper, S.M.; Bryant, P.A.; Donath, S.M.; Babl, F.E. Quick-Wee: A Novel Non-Invasive Urine Collection Method. Emerg. Med. J. 2017, 34, 63–64. [Google Scholar] [CrossRef]

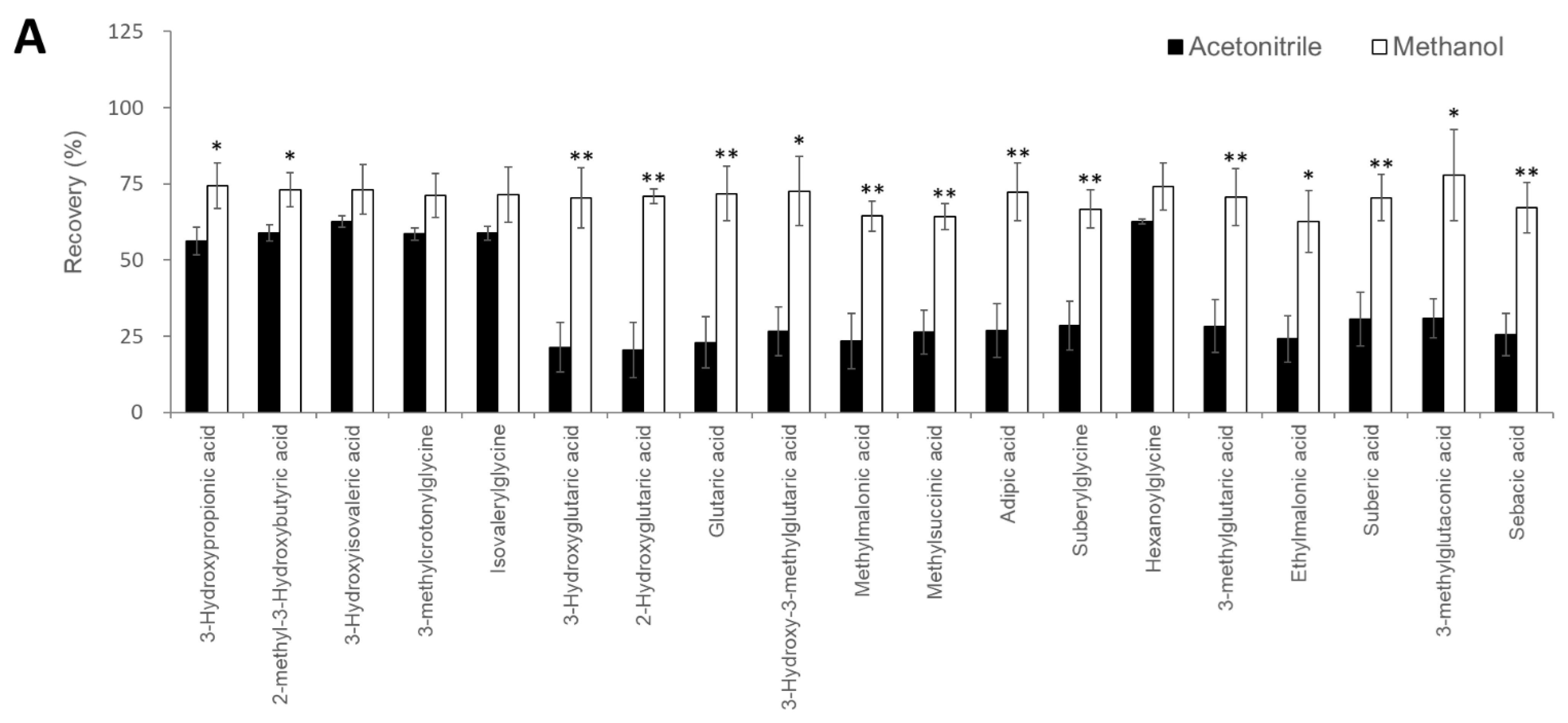

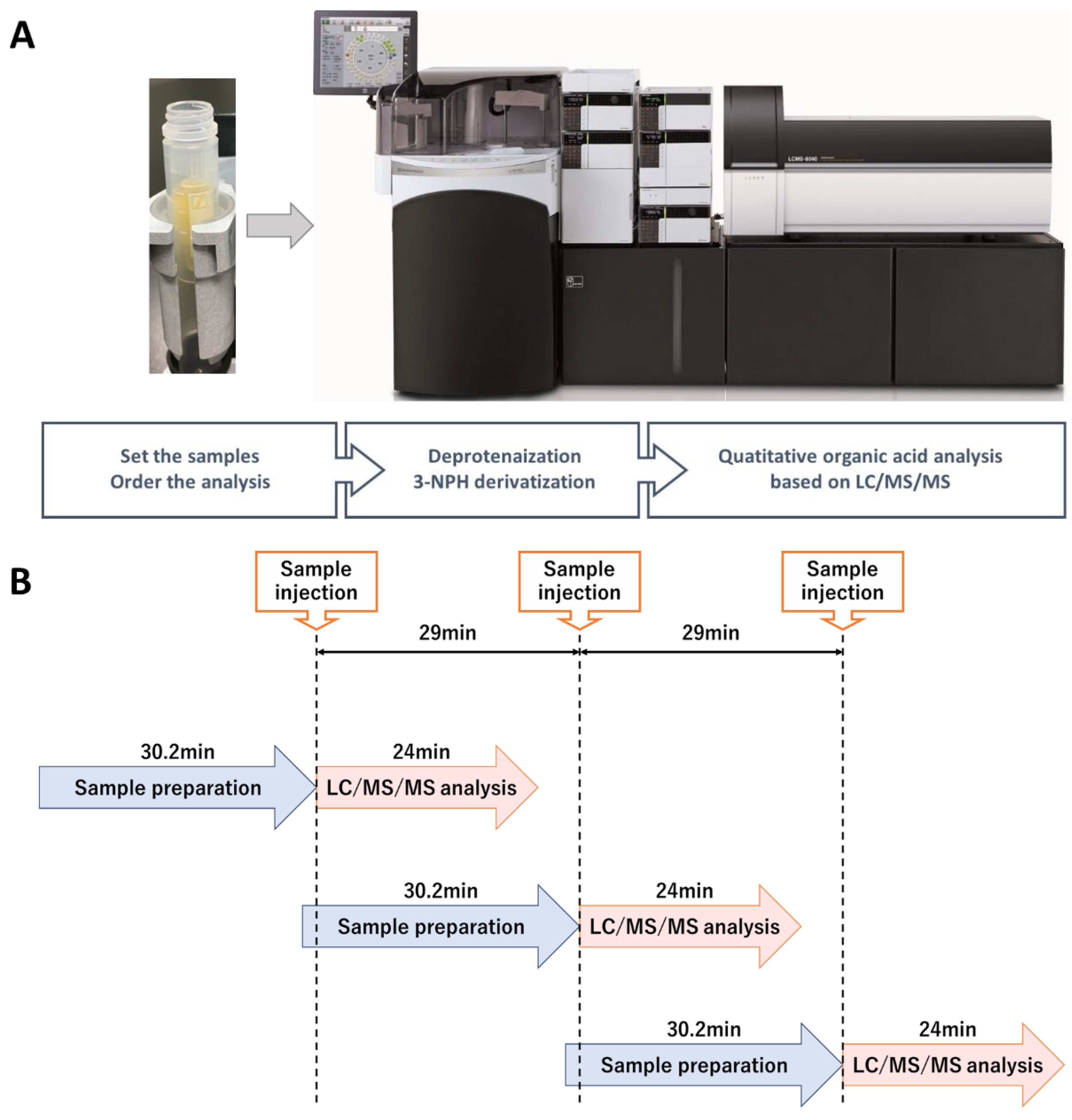
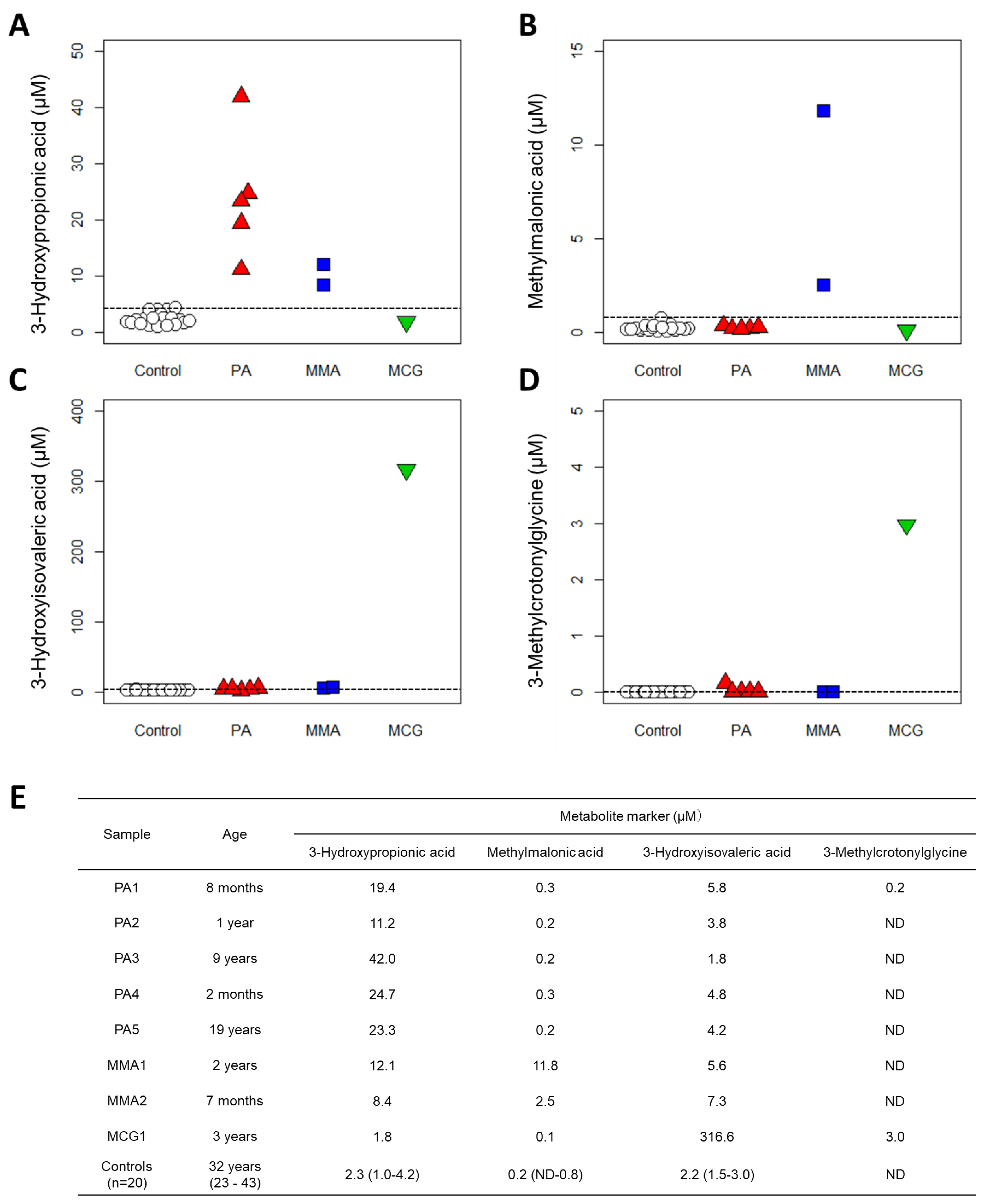
| Compounds | Number of Carboxyl Groups | Number of 3-NPH Derivatized | Precursor Ion (m/z) | Product Ion (m/z) | Retention Time (min) | |
|---|---|---|---|---|---|---|
| Quantitation | Reference | |||||
| 3-Hydroxypropionic acid | 1 | 1 | 224.2 | 194.1 | 152.0 | 3.82 |
| 2-Methyl-3-hydroxybutyric acid | 1 | 1 | 252.0 | 208.1 | 165.1 | 6.75 |
| 3-Hydroxyisovaleric acid | 1 | 1 | 252.2 | 194.0 | 152.0 | 7.23 |
| 3-Methylcrotonylglycine | 1 | 1 | 291.0 | 209.0 | 137.0 | 10.11 |
| Isovalerylglycine | 1 | 1 | 293.0 | 137.0 | 209.0 | 10.30 |
| 3-Hydroxyglutaric acid | 2 | 2 | 417.0 | 222.0 | 178.0 | 10.87 |
| 2-Hydroxyglutaric acid | 2 | 2 | 417.0 | 137.0 | 220.0 | 11.33 |
| Glutaric acid | 2 | 2 | 401.0 | 152.0 | 248.0 | 12.08 |
| 3-Hydroxy-3-methylglutaric acid | 2 | 2 | 431.0 | 236.1 | 178.0 | 12.19 |
| Methylmalonic acid | 2 | 2 | 387.0 | 178.0 | 150.0 | 12.22 |
| Methylsuccinic acid | 2 | 2 | 401.1 | 248.0 | 111.9 | 12.28 |
| Adipic acid | 2 | 2 | 415.0 | 178.0 | 262.1 | 12.42 |
| Suberylglycine | 2 | 2 | 500.2 | 137.0 | 209.1 | 12.44 |
| Hexanoylglycine | 1 | 1 | 307.1 | 137.0 | 208.9 | 12.49 |
| 3-Methylglutaric acid | 2 | 2 | 415.1 | 262.1 | 152.0 | 12.80 |
| Ethylmalonic acid | 2 | 2 | 401.1 | 178.0 | 150.0 | 13.29 |
| Suberic acid | 2 | 2 | 443.2 | 152.0 | 137.0 | 13.71 |
| 3-Methylglutaconic acid | 2 | 2 | 413.0 | 260.0 | 178.0 | 13.72 |
| Sebacic acid | 2 | 2 | 471.1 | 137.0 | 152.0 | 15.23 |
| 13C3-3-Hydroxypropionic acid (ISTD) | 1 | 1 | 226.8 | 196.0 | 152.0 | 3.82 |
| 13C4-Methylmalonic acid (ISTD) | 2 | 2 | 391.2 | 151.0 | 179.1 | 12.22 |
| 2-Ethylbutyric acid (ISTD) | 1 | 1 | 250.2 | 137.0 | 107.0 | 13.71 |
| Compounds | r2 | LLOD (µM) | Accuracy (%) | Intra-Assay (n = 5) (CV%) | Inter-Assay (n = 5) (CV%) | |||
|---|---|---|---|---|---|---|---|---|
| 5 µM | 25 µM | 5 µM | 25 µM | 5 µM | 25 µM | |||
| 3-Hydroxypropionic acid | 0.998 | 0.13 | 113.6 | 96.8 | 0.9 | 1.8 | 4.5 | 4.6 |
| 2-Methyl-3-hydroxybutyric acid | 0.998 | 0.06 | 100.8 | 93.7 | 4.3 | 4.6 | 3.1 | 4.6 |
| 3-Hydroxyisovaleric acid | 0.996 | 0.25 | 99.9 | 95.4 | 5.5 | 5.3 | 3.4 | 3.6 |
| 3-Methylcrotonylglycine | 0.996 | 0.06 | 100 | 94.9 | 1.8 | 2.0 | 5.6 | 4.3 |
| Isovalerylglycine | 0.997 | 0.06 | 101.6 | 100.6 | 3.4 | 3.4 | 5.2 | 3.5 |
| 3-Hydroxyglutaric acid | 0.994 | 0.06 | 96.3 | 94.0 | 3.7 | 1.8 | 5.3 | 3.6 |
| 2-Hydroxyglutaric acid | 0.998 | 0.06 | 96.5 | 92.5 | 4.6 | 1.7 | 4.1 | 5.7 |
| Glutaric acid | 0.999 | 0.13 | 101.5 | 97.4 | 3.9 | 1.8 | 8.6 | 5.5 |
| 3-Hydroxy-3-methylglutaric acid | 0.998 | 0.06 | 87.2 | 94.7 | 6.0 | 3.6 | 9.3 | 3.7 |
| Methylmalonic acid | 0.999 | 0.06 | 110.4 | 97.2 | 4.8 | 5.2 | 9.0 | 4.9 |
| Methylsuccinic acid | 0.997 | 0.50 | 102.8 | 101.0 | 6.5 | 2.3 | 4.0 | 1.8 |
| Adipic acid | 0.997 | 0.13 | 103.9 | 96.4 | 5.1 | 2.0 | 7.9 | 4.1 |
| Suberylglycine | 0.999 | 0.06 | 108.9 | 103.1 | 2.1 | 4.1 | 10.4 | 3.0 |
| Hexanoylglycine | 0.998 | 0.06 | 109.0 | 100.3 | 4.2 | 3.6 | 5.3 | 3.2 |
| 3-Methylglutaric acid | 0.999 | 0.06 | 105.0 | 100.1 | 2.9 | 1.9 | 3.7 | 2.2 |
| Ethylmalonic acid | 0.992 | 0.13 | 94.7 | 99.5 | 3.0 | 3.9 | 10.2 | 5.6 |
| Suberic acid | 0.997 | 0.06 | 114.1 | 100.6 | 2.3 | 4.3 | 6.0 | 2.3 |
| 3-Methylglutaconic acid | 0.999 | 0.06 | 106.3 | 97.8 | 3.3 | 3.5 | 7.1 | 3.5 |
| Sebacic acid | 0.999 | 0.50 | 109.6 | 99.5 | 2.2 | 3.5 | 8.8 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueyanagi, Y.; Setoyama, D.; Kawakami, D.; Mushimoto, Y.; Matsumoto, S.; Hotta, T.; Kang, D. Fully Automated Quantitative Measurement of Serum Organic Acids via LC-MS/MS for the Diagnosis of Organic Acidemias: Establishment of an Automation System and a Proof-of-Concept Validation. Diagnostics 2021, 11, 2195. https://doi.org/10.3390/diagnostics11122195
Ueyanagi Y, Setoyama D, Kawakami D, Mushimoto Y, Matsumoto S, Hotta T, Kang D. Fully Automated Quantitative Measurement of Serum Organic Acids via LC-MS/MS for the Diagnosis of Organic Acidemias: Establishment of an Automation System and a Proof-of-Concept Validation. Diagnostics. 2021; 11(12):2195. https://doi.org/10.3390/diagnostics11122195
Chicago/Turabian StyleUeyanagi, Yasushi, Daiki Setoyama, Daisuke Kawakami, Yuichi Mushimoto, Shinya Matsumoto, Taeko Hotta, and Dongchon Kang. 2021. "Fully Automated Quantitative Measurement of Serum Organic Acids via LC-MS/MS for the Diagnosis of Organic Acidemias: Establishment of an Automation System and a Proof-of-Concept Validation" Diagnostics 11, no. 12: 2195. https://doi.org/10.3390/diagnostics11122195
APA StyleUeyanagi, Y., Setoyama, D., Kawakami, D., Mushimoto, Y., Matsumoto, S., Hotta, T., & Kang, D. (2021). Fully Automated Quantitative Measurement of Serum Organic Acids via LC-MS/MS for the Diagnosis of Organic Acidemias: Establishment of an Automation System and a Proof-of-Concept Validation. Diagnostics, 11(12), 2195. https://doi.org/10.3390/diagnostics11122195







