Application and Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio in Patients with Ischemic and Non-Ischemic Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohorts
2.2. Transthoracic Echocardiography
2.3. Cardiac MRI
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
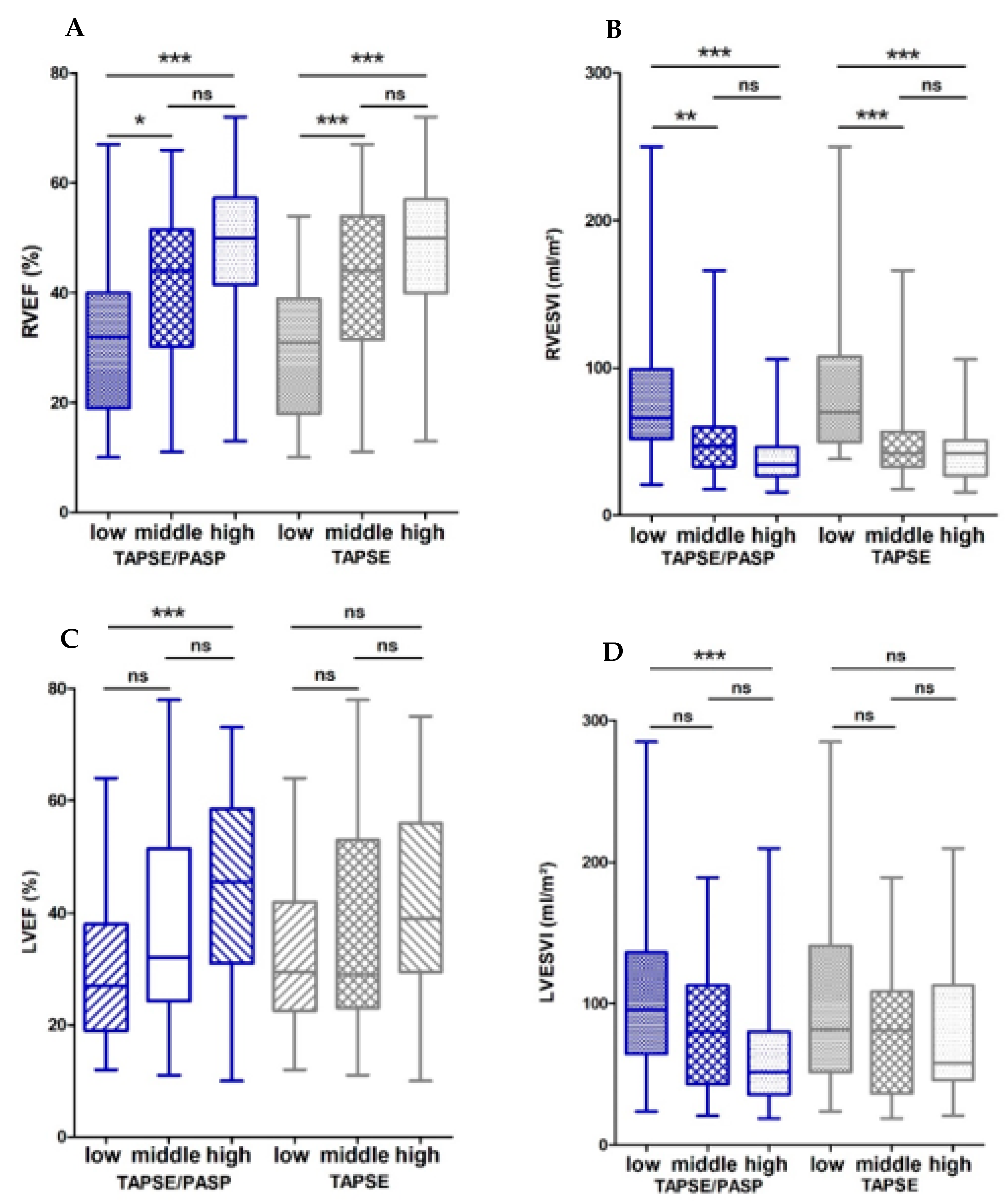
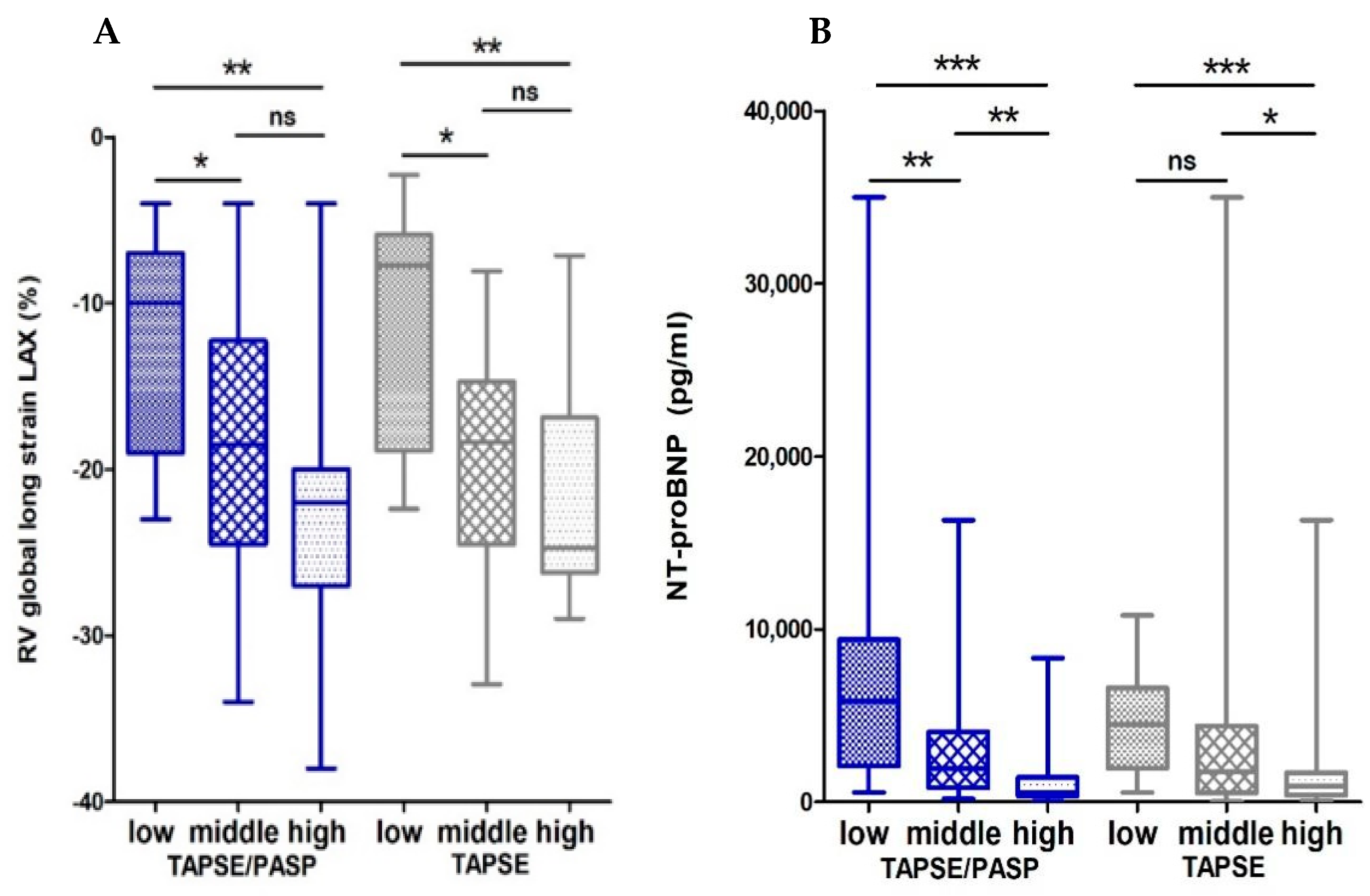
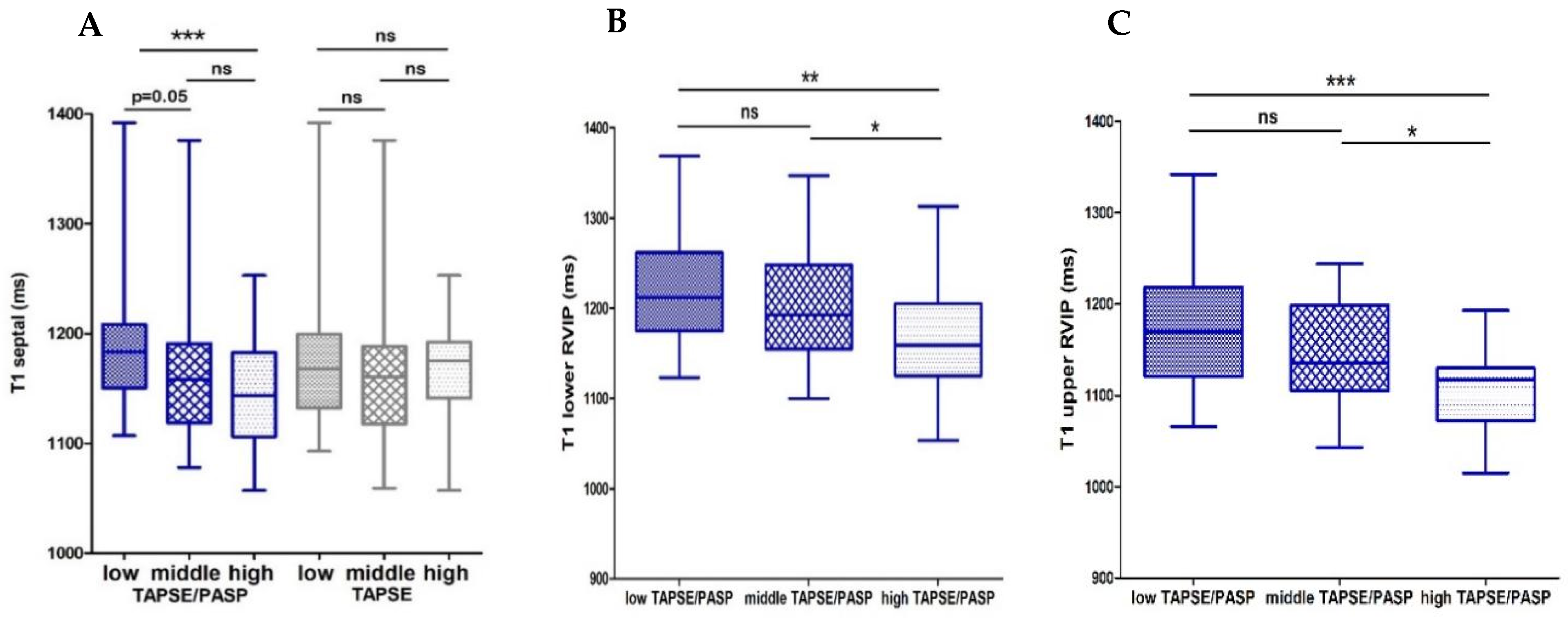
3.1.1. Associations of the Echocardiographic TAPSE/PASP Ratio and CMR
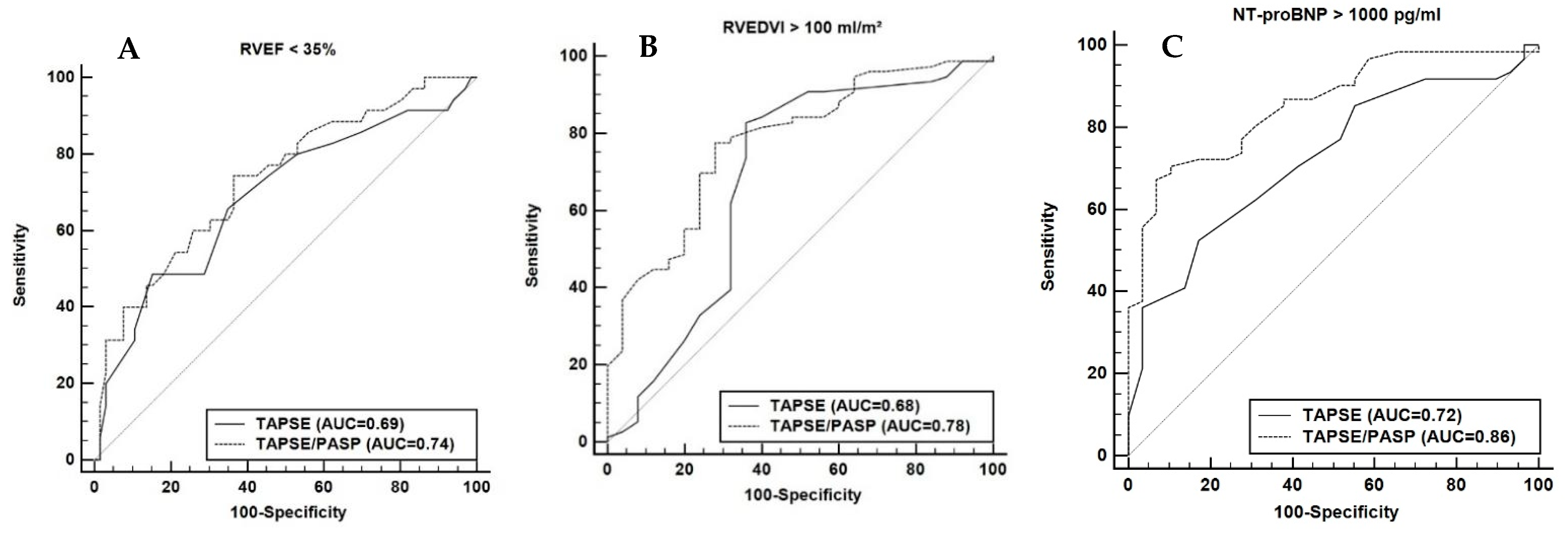
3.1.2. Echocardiographic TAPSE/PASP to Predict Maladaptive RV
3.1.3. Echocardiographic TAPSE/PASP as Predictor of Mortality
4. Discussion
- (1)
- Low TAPSE/PASP (cut-off 0.38 mm/mmHg) and a low TAPSE (cut-off 16 mm) are both associated with RV dysfunction, RV dilation and higher NT-proBNP levels;
- (2)
- TAPSE/PASP, but not TAPSE alone, shows an association with LV dysfunction, LV dilation and fibrosis in the ventricular septum and RV insertion points;
- (3)
- TAPSE/PASP < 0.38 mm/mmHg is associated with a significantly increased mortality and TAPSE/PASP is an independent predictor of mortality.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghio, S.; Temporelli, P.L.; Klersy, C.; Simioniuc, A.; Girardi, B.; Scelsi, L.; Rossi, A.; Cicoira, M.; Genta, F.T.; Dini, F.L. Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur. J. Heart Fail. 2013, 15, 408–414. [Google Scholar] [CrossRef]
- Ghio, S.; Gavazzi, A.; Campana, C.; Inserra, C.; Klersy, C.; Sebastiani, R.; Arbustini, E.; Recusani, F.; Tavazzi, L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001, 37, 183–188. [Google Scholar] [CrossRef]
- van der Bruggen, C.E.E.; Tedford, R.J.; Handoko, M.L.; van der Velden, J.; de Man, F.S. RV pressure overload: From hypertrophy to failure. Cardiovasc. Res. 2017, 113, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Wasemiller, S.; Earle, T.; Kashner, M.; Foster, G.; Silvet, H. Right Ventricular Ejection Fraction in Ischemic Versus Nonischemic Cardiomyopathy. Am. J. Cardiol. 2016, 117, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Moon, J.C.; Bellenger, N.G.; Smith, G.S.; Klein, H.U.; Pennell, D.J. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am. Heart J. 2004, 147, 218–223. [Google Scholar] [CrossRef]
- Mooij, C.F.; de Wit, C.J.; Graham, D.A.; Powell, A.J.; Geva, T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J. Magn. Reson. Imaging JMRI 2008, 28, 67–73. [Google Scholar] [CrossRef]
- Swift, A.J.; Capener, D.; Johns, C.; Hamilton, N.; Rothman, A.; Elliot, C.; Condliffe, R.; Charalampopoulos, A.; Rajaram, S.; Lawrie, A.; et al. Magnetic Resonance Imaging in the Prognostic Evaluation of Patients with Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2017, 196, 228–239. [Google Scholar] [CrossRef]
- Vonk Noordegraaf, A.; Chin, K.M.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef]
- Schalla, S.; Jaarsma, C.; Bekkers, S.C.; Waltenberger, J.; Dennert, R.; Crijns, H.J.; Wildberger, J.; Heymans, S.; Brunner-La Rocca, H.P. Right ventricular function in dilated cardiomyopathy and ischemic heart disease: Assessment with non-invasive imaging. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2015, 23, 232–240. [Google Scholar] [CrossRef][Green Version]
- Zornoff, L.A.; Skali, H.; Pfeffer, M.A.; St John Sutton, M.; Rouleau, J.L.; Lamas, G.A.; Plappert, T.; Rouleau, J.R.; Moyé, L.A.; Lewis, S.J.; et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J. Am. Coll. Cardiol. 2002, 39, 1450–1455. [Google Scholar] [CrossRef]
- Gulati, A.; Ismail, T.F.; Jabbour, A.; Alpendurada, F.; Guha, K.; Ismail, N.A.; Raza, S.; Khwaja, J.; Brown, T.D.; Morarji, K.; et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013, 128, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Sabe, M.A.; Sabe, S.A.; Kusunose, K.; Flamm, S.D.; Griffin, B.P.; Kwon, D.H. Predictors and Prognostic Significance of Right Ventricular Ejection Fraction in Patients with Ischemic Cardiomyopathy. Circulation 2016, 134, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Bourantas, C.V.; Loh, H.P.; Bragadeesh, T.; Rigby, A.S.; Lukaschuk, E.I.; Garg, S.; Tweddel, A.C.; Alamgir, F.M.; Nikitin, N.P.; Clark, A.L.; et al. Relationship between right ventricular volumes measured by cardiac magnetic resonance imaging and prognosis in patients with chronic heart failure. Eur. J. Heart Fail. 2011, 13, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bosch, L.; Lam, C.S.; Gong, L.; Chan, S.P.; Sim, D.; Yeo, D.; Jaufeerally, F.; Leong, K.T.G.; Ong, H.Y.; Ng, T.P.; et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1664–1671. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- von Jeinsen, B.; Ritzen, L.; Vietheer, J.; Unbehaun, C.; Weferling, M.; Liebetrau, C.; Hamm, C.W.; Rolf, A.; Keller, T. The adipokine fatty-acid binding protein 4 and cardiac remodeling. Cardiovasc. Diabetol. 2020, 19, 117. [Google Scholar] [CrossRef]
- Elsner, L.K.; von Jeinsen, B.V.; Grün, D.; Wolter, J.S.; Weferling, M.; Diouf, K.; Kriechbaum, S.; Troidl, C.; Dörr, O.; Nef, H.; et al. Prognostic performance of the ESC SCORE and its German recalibrated versions in primary and secondary prevention. Eur. J. Prev. Cardiol. 2020, 27, 2166–2169. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.Y.; Gebker, R.; Kelle, S.; Hinojar, R.; Doltra, A.; Varma, N.; Child, N.; et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovasc. Imaging 2016, 9, 40–50. [Google Scholar] [CrossRef]
- Geva, T. Is MRI the preferred method for evaluating right ventricular size and function in patients with congenital heart disease?: MRI is the preferred method for evaluating right ventricular size and function in patients with congenital heart disease. Circ. Cardiovasc. Imaging 2014, 7, 190–197. [Google Scholar] [CrossRef]
- Agasthi, P.; Chao, C.J.; Siegel, R.J.; Pujari, S.H.; Mookadam, F.; Venepally, N.R.; Wang, P.; Ashraf, H.; Marcotte, F.; Brown, L.; et al. Comparison of echocardiographic parameters with cardiac magnetic resonance imaging in the assessment of right ventricular function. Echocardiography 2020, 37, 1792–1802. [Google Scholar] [CrossRef]
- Beygui, F.; Furber, A.; Delépine, S.; Helft, G.; Metzger, J.P.; Geslin, P.; Le Jeune, J.J. Routine breath-hold gradient echo MRI-derived right ventricular mass, volumes and function: Accuracy, reproducibility and coherence study. Int. J. Cardiovasc. Imaging 2004, 20, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Larose, E.; Ganz, P.; Reynolds, H.G.; Dorbala, S.; Di Carli, M.F.; Brown, K.A.; Kwong, R.Y. Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction. J. Am. Coll. Cardiol. 2007, 49, 855–862. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, M.C.; Kind, T.; Marcus, J.T.; Mauritz, G.J.; Heymans, M.W.; Bogaard, H.J.; Boonstra, A.; Marques, K.M.; Westerhof, N.; Vonk-Noordegraaf, A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J. Am. Coll. Cardiol. 2011, 58, 2511–2519. [Google Scholar] [CrossRef]
- Swift, A.J.; Rajaram, S.; Campbell, M.J.; Hurdman, J.; Thomas, S.; Capener, D.; Elliot, C.; Condliffe, R.; Wild, J.M.; Kiely, D.G. Prognostic value of cardiovascular magnetic resonance imaging measurements corrected for age and sex in idiopathic pulmonary arterial hypertension. Circ. Cardiovasc. Imaging 2014, 7, 100–106. [Google Scholar] [CrossRef]
- Swift, A.J.; Rajaram, S.; Campbell, M.J.; Hurdman, J.; Thomas, S.; Capener, D.; Elliot, C.; Condliffe, R.; Wild, J.M.; Kiely, D.G. Prognostic value of right ventricular ejection fraction in pulmonary arterial hypertension. Eur. Respir. J. 2015, 45, 139–149. [Google Scholar]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Schmeisser, A.; Rauwolf, T.; Groscheck, T.; Kropf, S.; Luani, B.; Tanev, I.; Hansen, M.; Meißler, S.; Steendijk, P.; Braun-Dullaeus, R.C. Pressure-volume loop validation of TAPSE/PASP for right ventricular arterial coupling in heart failure with pulmonary hypertension. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 168–176. [Google Scholar] [CrossRef]
- Ghio, S.; Guazzi, M.; Scardovi, A.B.; Klersy, C.; Clemenza, F.; Carluccio, E.; Temporelli, P.L.; Rossi, A.; Faggiano, P.; Traversi, E.; et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur. J. Heart Fail. 2017, 19, 873–879. [Google Scholar] [CrossRef]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef]
- Freed, B.H.; Gomberg-Maitland, M.; Chandra, S.; Mor-Avi, V.; Rich, S.; Archer, S.L.; Jamison, E.B.; Lang, R.M.; Patel, A.R. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J. Cardiovasc. Magn. Reson. 2012, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.I.; Bogaard, H.J.; Mizuno, S.; Clifton, B.; Xie, B.; Gao, Y.; Dumur, C.I.; Fawcett, P.; Voelkel, N.F.; Natarajan, R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Fibroblasts and the extracellular matrix in right ventricular disease. Cardiovasc. Res. 2017, 113, 1453–1464. [Google Scholar] [CrossRef]
- Rain, S.; Handoko, M.L.; Trip, P.; Gan, C.T.J.; Westerhof, N.; Stienen, G.J.; Paulus, W.J.; Ottenheijm, C.A.; Marcus, J.T.; Dorfmüller, P.; et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 2013, 128, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Trip, P.; Rain, S.; Handoko, M.L.; Van der Bruggen, C.; Bogaard, H.J.; Marcus, J.T.; Boonstra, A.; Westerhof, N.; Vonk-Noordegraaf, A.; Frances, S. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur. Respir. J. 2015, 45, 1603–1612. [Google Scholar] [CrossRef]
- García-Álvarez, A.; García-Lunar, I.; Pereda, D.; Fernández-Jimenez, R.; Sánchez-González, J.; Mirelis, J.G.; Nuño-Ayala, M.; Sánchez-Quintana, D.; Fernández-Friera, L.; García-Ruiz, J.M.; et al. Association of myocardial T1-mapping CMR with hemodynamics and RV performance in pulmonary hypertension. JACC Cardiovasc. Imaging 2015, 8, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Nielsen-Kudsk, J.E.; Vonk Noordegraaf, A.; de Man, F.S. Right Ventricular Fibrosis. Circulation 2019, 139, 269–285. [Google Scholar] [CrossRef]
- Roller, F.C.; Wiedenroth, C.; Breithecker, A.; Liebetrau, C.; Mayer, E.; Schneider, C.; Rolf, A.; Hamm, C.; Krombach, G.A. Native T1 mapping and extracellular volume fraction measurement for assessment of right ventricular insertion point and septal fibrosis in chronic thromboembolic pulmonary hypertension. Eur. Radiol. 2017, 27, 1980–1991. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev. Esp. Cardiol. 2016, 69, 177. [Google Scholar] [CrossRef]

| Data Availability | n = 111 | |
|---|---|---|
| Age, y, median (IQR) | 111/111 | 69 (60–76) |
| Female sex, n, (%) | 111/111 | 32 (29) |
| BMI, kg/m2, mean (±SD) | 110/111 | 27 ± 4 |
| BSA, m2, mean (±SD) | 110/111 | 1.99 ± 0.22 |
| Cardiovascular risk factors | ||
| Hypertension, n, (%) | 109/111 | 83 (76) |
| Diabetes, n, (%) | 109/111 | 35 (32) |
| Insulin, n, (%) | 109/111 | 10 (9) |
| Dyslipidemia, n, (%) | 105/111 | 59 (56) |
| Clinical history | ||
| Chronic pulmonary disease, n, (%) | 109/111 | 17 (16) |
| CAD, n, (%) | 111/111 | 67 (60) |
| Prior MI, n, (%) | 104/111 | 37 (36) |
| Prior PCI, n, (%) | 108/111 | 41 (38) |
| Prior CABG, n, (%) | 110/111 | 21 (19) |
| Prior stroke/TIA, n, (%) | 109/111 | 17 (16) |
| Peripheral artery disease, n, (%) | 109/111 | 10 (9) |
| Symptoms | ||
| NYHA >/= 3, n, (%) | 111/111 | 42 (49) |
| Syncope, n, (%) | 109/111 | 8 (7) |
| Prior cardiac decompensation, n, (%) | 47/111 | 26 (55) |
| Echocardiographic findings | ||
| LVEF, %, median (IQR) | 111/111 | 35 (25–51) |
| LVEF < 40%, n, (%) | 111/111 | 71 (64) |
| LVEDd, mm, mean (±SD) | 100/111 | 56 ± 9 |
| LA, mm, median (IQR) | 96/111 | 43 (38–46) |
| IVSd, mm, median (IQR) | 100/111 | 10 (9–12) |
| LVPWd, mm, median (IQR) | 98/111 | 10 (9–11) |
| RVEDd, mm, median (IQR) | 96/111 | 35 (32–40) |
| TAPSE, mm, mean (±SD) | 111/111 | 18 ± 5 |
| PASP, mmHg, median (IQR) | 111/111 | 39 (30–46) |
| TAPSE/PASP, mm/mmHg, median (IQR) | 111/111 | 0.46 (0.34–0.66) |
| AS >/= II, n, (%) | 107/111 | 3 (3) |
| MI >/= II, n, (%) | 107/111 | 17 (16) |
| Biomarker | ||
| Creatinine, mmol/L, median (IQR) | 105/111 | 0.92 (0.77–1.26) |
| eGFR, mL/min/1.73 m², mean (±SD) | 102/111 | 81 ± 28 |
| NT–proBNP, pg/mL, median (IQR) | 90/111 | 1697 (555–4524) |
| MRI findings | ||
| HF, 1/min, median (IQR) | 105/111 | 70 (64–80) |
| LVEF, %, median (IQR) | 107/111 | 35 (24–50) |
| LVEDV, mL, median (IQR) | 101/111 | 216 (168–308) |
| LVEDVI, mL/m2, median (IQR) | 101/111 | 108 (86–149) |
| LVESV, mL, median (IQR) | 101/111 | 75 (47–113) |
| LVESVI, mL/m2, median (IQR) | 101/111 | 140 (88–232) |
| LA area, m2, mean (± SD) | 84/111 | 28 ± 8 |
| RVEF, %, median (IQR) | 101/111 | 40 (27–51) |
| RVEDV, mL, median (IQR) | 101/111 | 158 (123–202) |
| RVEDVI, mL/m2, median (IQR) | 101/111 | 78 (70–99) |
| RV ESVI, mL/m2, median (IQR) | 101/111 | 47 (34–67) |
| RV ESV, ml, median (IQR), ml, median (IQR) | 101/111 | 94 (68–138) |
| RV SV, mL, median (IQR) | 101/111 | 64 (49–79) |
| RA area, m2, median (IQR) | 85/111 | 26 (23–32) |
| CI, l/min × m2, median (IQR) | 101/111 | 2.6 (2.1–3.2) |
| LV longitudinal Strain LAX global, %, median (IQR) | 44/111 | −10 (−14; −7) |
| RV longitudinal Strain LAX global, %, median (IQR) | 44/111 | −18 (−24; −10) |
| T1 time septal, s, median (IQR) | 102/111 | 1165 (1132–1191) |
| T1 time upper RVIP, s, median (IQR) | 96/111 | 1129 (1102–1181) |
| T1 time lower RVIP, s, median (IQR) | 96/111 | 1190 (1142–1232) |
| TAPSE/PASP r | TAPSE r | TAPSE/PASP vs. TAPSE (p) | |
|---|---|---|---|
| MRI findings | |||
| LVEF | 0.32 ** | 0.18 | 0.04 |
| LV-Masse | −0.29 ** | −0.13 | 0.02 |
| LVEDVI | −0.28 ** | −0.02 | <0.001 |
| LVESVI | −0.31 ** | −0.09 | 0.001 |
| LA area | −0.34 ** | −0.19 | 0.02 |
| RVEF | 0.45 ** | 0.38 ** | 0.26 |
| RVEDVI, mL/m2 | −0.42 ** | −0.32 ** | 0.12 |
| RVESVI, mL/m2 | −0.52 ** | −0.40 ** | 0.049 |
| RA area | −0.21 | −0.15 | 0.38 |
| RV global longitudinal Strain LAX | −0.51 ** | −0.45 ** | 0.49 |
| LV global longitudinal Strain LAX | −0.40 ** | −0.28 * | 0.23 |
| T1 septal | −0.22 * | −0.07 | 0.03 |
| T1 Upper RVIP | −0.37 ** | −0.19 | 0.007 |
| T1 Lower RVIP | −0.32 ** | −0.13 | 0.005 |
| Cardiac Index | −0.02 | 0.04 | 0.39 |
| Biomarkers | |||
| NT-pro-BNP | −0.70 ** | −0.42 ** | <0.001 |
| eGFR | 0.26 ** | 0.21 * | 0.46 |
| Data Availability | All | Non Survivors | Survivors | p-Value | |
|---|---|---|---|---|---|
| n = 221 | n = 89 | n = 132 | |||
| Non-survivors, n, (%) | 221/221 | 89 (40) | 89 (100) | 0 (0) | |
| Age, y, median (IQR) | 221/221 | 72 (64–78) | 73 (68–78) | 70 (61–77) | 0.03 |
| Female sex, n, (%) | 221/221 | 43 (19) | 12 (13) | 31 (23) | 0.08 |
| BMI, kg/m2, median (IQR) | 221/221 | 27 (25–31) | 27 (25–31) | 27 (24–31) | 0.73 |
| BSA, m2, median (IQR) | 221/221 | 1.99 (1.83–2.15) | 2 (1.84–2.11) | 1.98 (1.83–2.17) | 0.97 |
| Cardiovascular risk factors | |||||
| Hypertension, n, (%) | 221/221 | 179 (81) | 73 (82) | 106 (80) | 0.86 |
| Diabetes, n, (%) | 221/221 | 69 (31) | 36 (40) | 33 (25) | 0.02 |
| Dyslipidemia, n, (%) | 221/221 | 145 (66) | 57 (64) | 88 (67) | 0.77 |
| Clinical history | |||||
| CAD, n, (%) | 221/221 | 137 (62) | 65 (73) | 72 (55) | 0.01 |
| Prior MI, n, (%) | 221/221 | 75 (34) | 37 (42) | 38 (29) | 0.06 |
| Prior PCI, n, (%) | 221/221 | 85 (38) | 45 (51) | 40 (30) | 0.003 |
| Prior CABG, n, (%) | 221/221 | 47 (21) | 25 (28) | 22 (17) | 0.05 |
| Prior stroke/TIA, n, (%) | 216/221 | 27 (13) | 15 (17) | 12 (9) | 0.10 |
| Peripheral artery disease, n, (%) | 216/221 | 21 (10) | 12 (14) | 9 (7) | 0.11 |
| Symptoms | |||||
| NYHA >/= 3, n, (%) | 221/221 | 113 (58) | 48 (59) | 65 (57) | 0.58 |
| Syncope, n, (%) | 200/221 | 12 (6) | 5 (6) | 7 (6) | 0.98 |
| Echocardiographic findings | |||||
| LVEF, %, median (IQR) | 221/221 | 30 (25–36) | 30 (25–35) | 34 (25–40) | 0.001 |
| LVEF < 35%, n, (%) | 221/221 | 130 (59) | 63 (71) | 67 (51) | 0.003 |
| LVEDd, mm, mean (±SD) | 213/221 | 60 ± 8 | 62 ± 8 | 58 ± 8 | 0.003 |
| LA, mm, median (IQR) | 213/221 | 45 (42–50) | 46 (42–50) | 45 (42–50) | 0.92 |
| IVSd, mm, median (IQR) | 206/221 | 11 (10–12) | 11 (10–12) | 11 (10–12) | 0.93 |
| LVPWd, mm, median (IQR) | 191/221 | 11 (10–12) | 11 (9–12) | 11 (10–12) | 0.22 |
| RVEDd, mm, median (IQR) | 185/221 | 33 (30–37) | 33 (30–36) | 33 (30–37) | 0.65 |
| TAPSE, mm, mean (±SD) | 221/221 | 18 (15–21) | 18 (15–21) | 17 (15–20) | 0.12 |
| PASP, mmHg, median (IQR) | 221/221 | 40 (32–53) | 44 (35–56) | 38 (31–50) | 0.002 |
| TAPSE/PASP, mm/mmHg, median (IQR) | 221/221 | 0.42 (0.32–0.57) | 0.38 (0.3–0.51) | 0.46 (0.35–0.63) | 0.002 |
| AS >= II, n, (%) | 190/221 | 26 (14) | 15 (19) | 11 (10) | 0.06 |
| MI >= II, n, (%) | 219/221 | 92 (42) | 32 (37) | 60 (45) | 0.17 |
| Biomarker | |||||
| Creatinine, mmol/L, median (IQR) | 220/221 | 1.02 (0.85–1.3) | 1.16 (0.94–1.4) | 1 (0.82–1.21) | 0.002 |
| GFR, ml/min, median (IQR) | 220/221 | 70 (55–92) | 66 (52–82) | 74 (59–96) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keranov, S.; Haen, S.; Vietheer, J.; Rutsatz, W.; Wolter, J.-S.; Kriechbaum, S.D.; von Jeinsen, B.; Bauer, P.; Tello, K.; Richter, M.; et al. Application and Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio in Patients with Ischemic and Non-Ischemic Cardiomyopathy. Diagnostics 2021, 11, 2188. https://doi.org/10.3390/diagnostics11122188
Keranov S, Haen S, Vietheer J, Rutsatz W, Wolter J-S, Kriechbaum SD, von Jeinsen B, Bauer P, Tello K, Richter M, et al. Application and Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio in Patients with Ischemic and Non-Ischemic Cardiomyopathy. Diagnostics. 2021; 11(12):2188. https://doi.org/10.3390/diagnostics11122188
Chicago/Turabian StyleKeranov, Stanislav, Saskia Haen, Julia Vietheer, Wiebke Rutsatz, Jan-Sebastian Wolter, Steffen D. Kriechbaum, Beatrice von Jeinsen, Pascal Bauer, Khodr Tello, Manuel Richter, and et al. 2021. "Application and Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio in Patients with Ischemic and Non-Ischemic Cardiomyopathy" Diagnostics 11, no. 12: 2188. https://doi.org/10.3390/diagnostics11122188
APA StyleKeranov, S., Haen, S., Vietheer, J., Rutsatz, W., Wolter, J.-S., Kriechbaum, S. D., von Jeinsen, B., Bauer, P., Tello, K., Richter, M., Dörr, O., Rieth, A. J., Nef, H., Hamm, C. W., Liebetrau, C., Rolf, A., & Keller, T. (2021). Application and Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio in Patients with Ischemic and Non-Ischemic Cardiomyopathy. Diagnostics, 11(12), 2188. https://doi.org/10.3390/diagnostics11122188






