Immunoreactivity of Polish Lyme Disease Patient Sera to Specific Borrelia Antigens—Part 1
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Group
2.2. ELISA Assay
2.3. Immunoblot Assay
2.4. Statistical Analysis
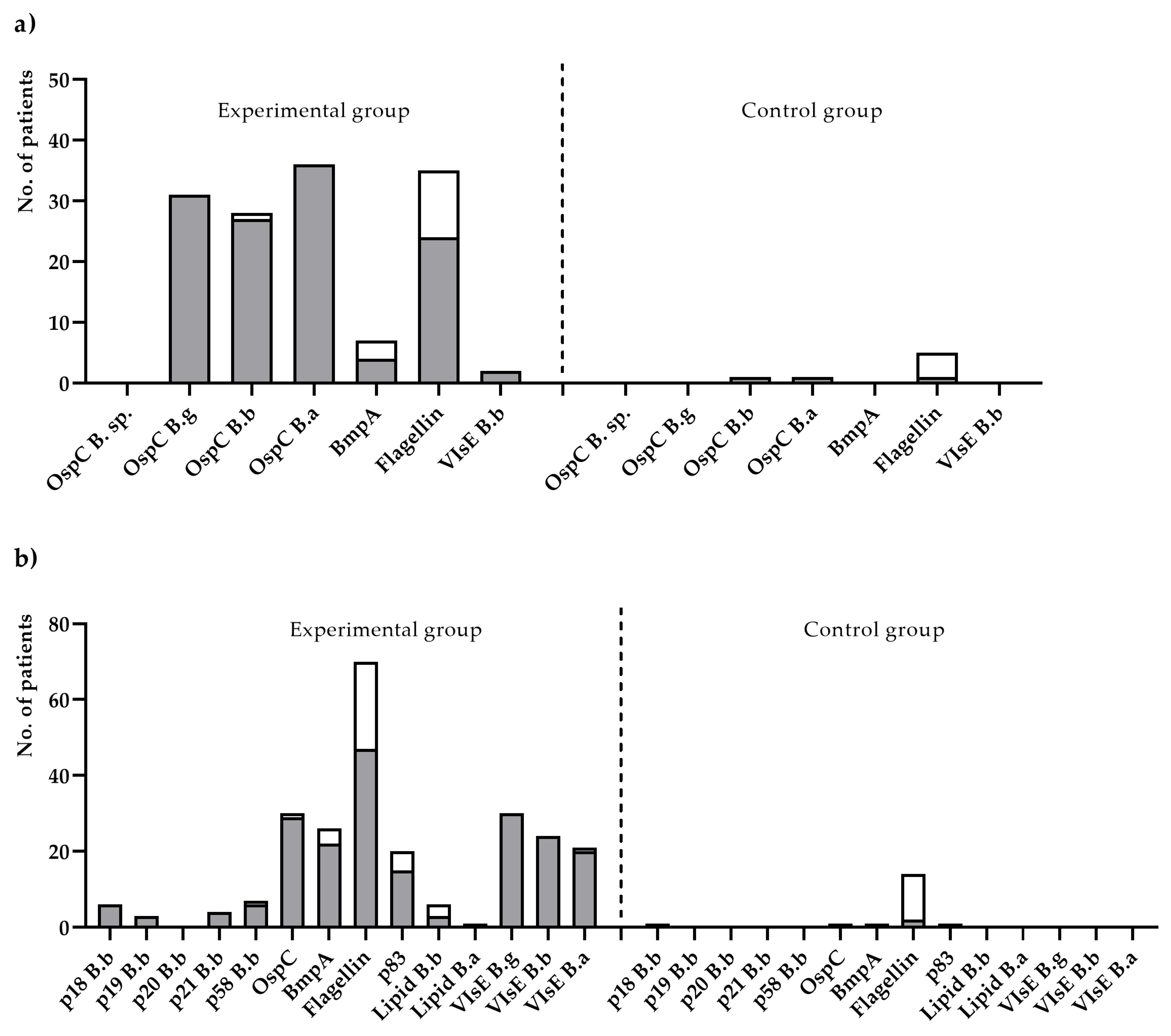
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Consent Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Cruz, I.D.; Ramos, C.C.; Taleon, P.; Ramasamy, R.; Shah, J. Pilot study of immunoblots with recombinant Borrelia burgdorferi antigens for laboratory diagnosis of Lyme disease. Healthcare 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilske, B.; Fingerle, V.; Schulte-Spechtel, U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol. Med. Microbiol. 2007, 49, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Wilske, B. Diagnosis of Lyme borreliosis in Europe. Vector Borne Zoonotic Dis. 2003, 3, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Biesiada, G.; Czepiel, J.; Salamon, D.; Garlicki, A.; Dziubek, A.; Maziarz, B.; Mach, T. Analysis of Borrelia burgdorferi genostrains among patients with Lyme disease. Przegl. Lek. 2009, 66, 511–512. [Google Scholar] [PubMed]
- Marques, A.R.; Strle, F.; Wormser, G.P. Comparison of Lyme disease in the United States and Europe. Emerg. Infect. Dis. 2021, 27, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Lindgren, E.; Jaenson, T. Lyme Borreliosis in Europe. Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2006; ISBN 92 890 2291 4. [Google Scholar]
- Øymar, K.; Tveitnes, D. Clinical characteristics of childhood Lyme neuroborreliosis in an endemic area of northern Europe. Scand. J. Infect. Dis. 2009, 41, 88–94. [Google Scholar] [CrossRef]
- Tveitnes, D.; Natås, O.B.; Skadberg, Ø.; Øymar, K. Lyme meningitis, the major cause of childhood meningitis in an endemic area: A population based study. Arch. Dis. Child. 2012, 97, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lipsker, D. Dermatological aspects of Lyme borreliosis. Med. Mal. Infect. 2007, 37, 540–547. [Google Scholar] [CrossRef]
- Theel, E.S. The past, present, and (possible) future of serologic testing for Lyme disease. J. Clin. Microbiol. 2016, 54, 1191–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguero-Rosenfeld, M.E.; Wang, G.; Schwartz, I.; Wormser, G.P. Diagnosis of Lyme borreliosis. Clin. Microbiol. Rev. 2005, 18, 484–509. [Google Scholar] [CrossRef] [Green Version]
- Lohr, B.; Fingerle, V.; Norris, D.E.; Hunfeld, K.-P. Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit. Rev. Clin. Lab. Sci. 2018, 55, 219–245. [Google Scholar] [CrossRef] [PubMed]
- CDC. Lyme Disease (Borrelia burgdorferi) 2017 Case Definition. Available online: https://ndc.services.cdc.gov/case-definitions/lyme-disease-2017/ (accessed on 30 September 2021).
- CDC. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR. Morb. Mortal. Wkly. Rep. 1995, 44, 590–591. [Google Scholar]
- Wojciechowska-Koszko, I.; Mnichowska-Polanowska, M. Serological testing for Lyme disease in laboratory practice. Postep. Mikrobiol. 2015, 54, 283–290. [Google Scholar]
- Ang, C.W.; Notermans, D.W.; Hommes, M.; Simoons-Smit, A.M.; Herremans, T. Large differences between test strategies for the detection of anti-Borrelia antibodies are revealed by comparing eight ELISAs and five immunoblots. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Latov, N.; Wormser, G.P.; Marques, A.R.; Alaedini, A. Epitope mapping of antibodies to VlsE protein of Borrelia burgdorferi in post-Lyme disease syndrome. Clin. Immunol. 2011, 141, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branda, J.A.; Linskey, K.; Kim, Y.A.; Steere, A.C.; Ferraro, M.J. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin. Infect. Dis. 2011, 53, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryksin, A.V.; Godfrey, H.P.; Carbonaro, C.A.; Wormser, G.P.; Aguero-Rosenfeld, M.E.; Cabello, F.C. Borrelia burgdorferi BmpA, BmpB, and BmpD proteins are expressed in human infection and contribute to P39 immunoblot reactivity in patients with Lyme disease. Clin. Diagn. Lab. Immunol. 2005, 12, 935–940. [Google Scholar] [CrossRef] [Green Version]
- Honegr, K.; Havlasová, J.; Gebouský, P.; Dostál, V.; Pellantová, V.; Skrabková, Z.; Hulínská, D. Criteria for evaluation of immunoblots using Borrelia afzelii, Borrelia garinii and Borrelia burgdorferi sensu stricto for diagnosis of Lyme borreliosis. Epidemiol. Mikrobiol. Imunol. 2001, 50, 147–156. [Google Scholar]
- Mavin, S.; Evans, R.; Milner, R.M.; Chatterton, J.M.W.; Ho-Yen, D.O. Local Borrelia burgdorferi sensu stricto and Borrelia afzelii strains in a single mixed antigen improves Western-blot sensitivity. J. Clin. Pathol. 2009, 62, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Lager, M.; Dessau, R.B.; Wilhelmsson, P.; Nyman, D.; Jensen, G.F.; Matussek, A.; Lindgren, P.-E.; Henningsson, A.J.; Baqir, H.; Serrander, L.; et al. Serological diagnostics of Lyme borreliosis: Comparison of assays in twelve clinical laboratories in Northern Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1933–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUROIMMUN Medizinische Labordiagnostika AG. Differentiated Borrelia Diagnostics. Available online: https://www.euroimmun.com/documents/Indications/Infections/Borrelia/HI_2132_I_UK_C.pdf. (accessed on 19 November 2021).
- Tracy, K.E.; Baumgarth, N. Borrelia burgdorferi manipulates innate and adaptive immunity to establish persistence in rodent reservoir hosts. Front. Immunol. 2017, 8, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Novoa, B.; Orduña, A.; Bratos, M.A.; Eiros, J.M.; Fernández, J.M.; Gutiérrez, M.P.; Alonso, P.A.; Mantecón, M.A.; Almaraz, A.; Oteo, J.A.; et al. Utility of a commercial immunoblot kit (BAG-Borrelia blot) in the diagnosis of the preliminary stages of Lyme disease. Diagn. Microbiol. Infect. Dis. 2003, 47, 321–329. [Google Scholar] [CrossRef]
- Goettner, G.; Schulte-Spechtel, U.; Hillermann, R.; Liegl, G.; Wilske, B.; Fingerle, V. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J. Clin. Microbiol. 2005, 43, 3602–3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, L.; Christova, I.; Neves, V.; Aroso, M.; Meirelles, L.; Brisson, D.; Gomes-Solecki, M. Comprehensive seroprofiling of sixteen B. burgdorferi OspC: Implications for Lyme disease diagnostics design. Clin. Immunol. 2009, 132, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Engstrom, S.M.; Shoop, E.; Johnson, R.C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 1995, 33, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Rauer, S.; Spohn, N.; Rasiah, C.; Neubert, U.; Vogt, A. Enzyme-linked immunosorbent assay using recombinant OspC and the internal 14-kDa flagellin fragment for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 1998, 36, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaboldi, P.M.; Seedarnee, R.; Sambir, M.; Callister, S.M.; Imparato, J.A.; Dattwyler, R.J. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early Lyme disease. Clin. Vaccine Immunol. 2013, 20, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Hubálek, Z.; Halouzka, J. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur. J. Epidemiol. 1997, 13, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Seppälä, I.; Saxen, H.; Panelius, J.; Yrjänäinen, H.; Lahdenne, P. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 2002, 40, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubiak, K.; Dzika, E.; Równiak, J.; Dziedziech, M.; Dzisko, J. Seroprevalence of Lyme disease and genospecies of Borrelia burgdorferi sensu lato in patients diagnosed with borreliosis in the Province of Warmia-Masuria in north-eastern Poland. Ann. Agric. Environ. Med. 2012, 19, 203–207. [Google Scholar]
- Cinco, M.; Murgia, R.; Ruscio, M.; Andriolo, B. IgM and IgG significant reactivity to Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii among Italian patients affected by Lyme arthritis or neuroborreliosis. FEMS Immunol. Med. Microbiol. 1996, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Strube, C.; Montenegro, V.M.; Epe, C.; Eckelt, E.; Schnieder, T. Establishment of a minor groove binder-probe based quantitative real time PCR to detect Borrelia burgdorferi sensu lato and differentiation of Borrelia spielmanii by OspA-specific conventional PCR. Parasit. Vectors 2010, 3, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauter, C.; Hartung, T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: A metaanalysis. Appl. Environ. Microbiol. 2005, 71, 7203–7216. [Google Scholar] [CrossRef] [Green Version]
- Chou, E.; Lin, Y.-P.; Cady, N.C. Recent strategies for the diagnosis of early Lyme disease. Sci. Prog. 2018, 101, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.S.F.; Anderson, K.W.; Benitez, K.Y.V.; Soloski, M.J.; Aucott, J.N.; Phinney, K.W.; Turko, I. V Quantification of Borrelia burgdorferi membrane proteins in human serum: A new concept for detection of bacterial infection. Anal. Chem. 2015, 87, 11383–11388. [Google Scholar] [CrossRef] [Green Version]
- Van Gorkom, T.; Voet, W.; Sankatsing, S.U.C.; Nijhuis, C.D.M.; Ter Haak, E.; Kremer, K.; Thijsen, S.F.T. Prospective comparison of two enzyme-linked immunosorbent spot assays for the diagnosis of Lyme neuroborreliosis. Clin. Exp. Immunol. 2020, 199, 337–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledue, T.B.; Collins, M.F.; Young, J.; Schriefer, M.E. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin. Diagn. Lab. Immunol. 2008, 15, 1796–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Serological Test | Result | Experimental Group n = 80 | Control Group n = 22 | ||||
|---|---|---|---|---|---|---|---|
| IgM n (%) | IgG n (%) | IgM and IgG n (%) | IgM n (%) | IgG n (%) | IgM and IgG n (%) | ||
| ELISA | Positive/borderline | 26 (32.5) | 11 (13.7) | 34 (42.5) | 2 (9.1) | 2 (9.1) | 0 (0.0) |
| IB | Positive/borderline | 12 (15.0) | 26 (32.5) | 27 (33.7) | 1 (4.5) | 1 (4.5) | 0 (0.0) |
| Class of Antibody/No. of Patients | Patient No. | ELISA | Immunoblot |
|---|---|---|---|
| IgM n = 5 | No. 24 | (-) | p41; OspC-adv B.a; OspC-adv B.b |
| No. 40 | (-) | p41; OspC-adv B.a | |
| No. 44 | (-) | p39 B.a; OspC-adv B.a; OspC-adv B.b | |
| No. 51 | (-) | p41; OspC-adv B.a | |
| No. 68 | (-) | p41; OspC-adv B.a | |
| IgG n = 15 | No. 9 | (-) | VlsE B.g; p41; p25 (OspC); p21 |
| No. 10 | (-) | p41; p25 (OspC) | |
| No.25 | (-) | p83 B.a; p41; p18 | |
| No. 30 | (-) | VlsE B.a; p41; p58; p21; p18 | |
| No. 36 | (-) | p83; p41 | |
| No. 37 | (-) | p41; p39; p25 (OspC) | |
| No. 43 | (-) | p41; p39; p25 (OspC); p19 | |
| No. 51 | (-) | p41; p39; p25 (OspC) | |
| No. 53 | (-) | p41; p39; p25 (OspC) | |
| No. 56 | (-) | VlsE B.b; p41 | |
| No. 60 | (-) | VlsE B.b; p41 | |
| No. 62 | (-) | p21 | |
| No. 65 | (-) | VlsE B.g; p83; p39 | |
| No. 75 | (-) | p83; p41; p39; p25 (OspC) | |
| No. 79 | (-) | Vls E B.g; VlsE B.a; p41; p39; p58 |
| Number of Genospecies | Genospecies | Experimental Group | Control Group | ||
|---|---|---|---|---|---|
| IgM n (%) | IgG n (%) | IgM n (%) | IgG n (%) | ||
| 1 | B.burgdorferi | 0 (0.0) | 6 (11.3) | 0 (0.0) | 1 (50.0) |
| B. garinii | 3 (7.6) | 7 (13.2) | 0 (0.0) | 0 (0.0) | |
| B. afzelii | 4 (10.3) | 1 (1.9) | 0 (0.0) | 0 (0.0) | |
| 2 | B.burgdorferi B. garinii | 0 (0.0) | 7 (13.2) | 0 (0.0) | 0 (0.0) |
| B.burgdorferi B. afzelii | 4 (10.3) | 1 (1.9) | 1 (100) | 0 (0.0) | |
| B. garinii B. afzelii | 4 (10.3) | 3 (5.7) | 0 (0.0) | 0 (0.0) | |
| 3 | B. burgdorferi B. garinii B. afzelii | 24 (61.5) | 15 (28.3) | 0 (0.0) | 0 (0.0) |
| Antigens common to all genospecies | 0 (0.0) | 13 (24.5) | 0 (0.0) | 1 (50.0) | |
| Total | 39 (100) | 53 (100) | 1 (100) | 2 (100) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowska-Koszko, I.; Mnichowska-Polanowska, M.; Kwiatkowski, P.; Roszkowska, P.; Sienkiewicz, M.; Dołęgowska, B. Immunoreactivity of Polish Lyme Disease Patient Sera to Specific Borrelia Antigens—Part 1. Diagnostics 2021, 11, 2157. https://doi.org/10.3390/diagnostics11112157
Wojciechowska-Koszko I, Mnichowska-Polanowska M, Kwiatkowski P, Roszkowska P, Sienkiewicz M, Dołęgowska B. Immunoreactivity of Polish Lyme Disease Patient Sera to Specific Borrelia Antigens—Part 1. Diagnostics. 2021; 11(11):2157. https://doi.org/10.3390/diagnostics11112157
Chicago/Turabian StyleWojciechowska-Koszko, Iwona, Magdalena Mnichowska-Polanowska, Paweł Kwiatkowski, Paulina Roszkowska, Monika Sienkiewicz, and Barbara Dołęgowska. 2021. "Immunoreactivity of Polish Lyme Disease Patient Sera to Specific Borrelia Antigens—Part 1" Diagnostics 11, no. 11: 2157. https://doi.org/10.3390/diagnostics11112157
APA StyleWojciechowska-Koszko, I., Mnichowska-Polanowska, M., Kwiatkowski, P., Roszkowska, P., Sienkiewicz, M., & Dołęgowska, B. (2021). Immunoreactivity of Polish Lyme Disease Patient Sera to Specific Borrelia Antigens—Part 1. Diagnostics, 11(11), 2157. https://doi.org/10.3390/diagnostics11112157