Multimer Analysis of Von Willebrand Factor in Von Willebrand Disease with a Hydrasys Semi-Automatic Analyzer—Single-Center Experience
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Electrophoresis
2.3. Immunofixation
2.4. Visualization
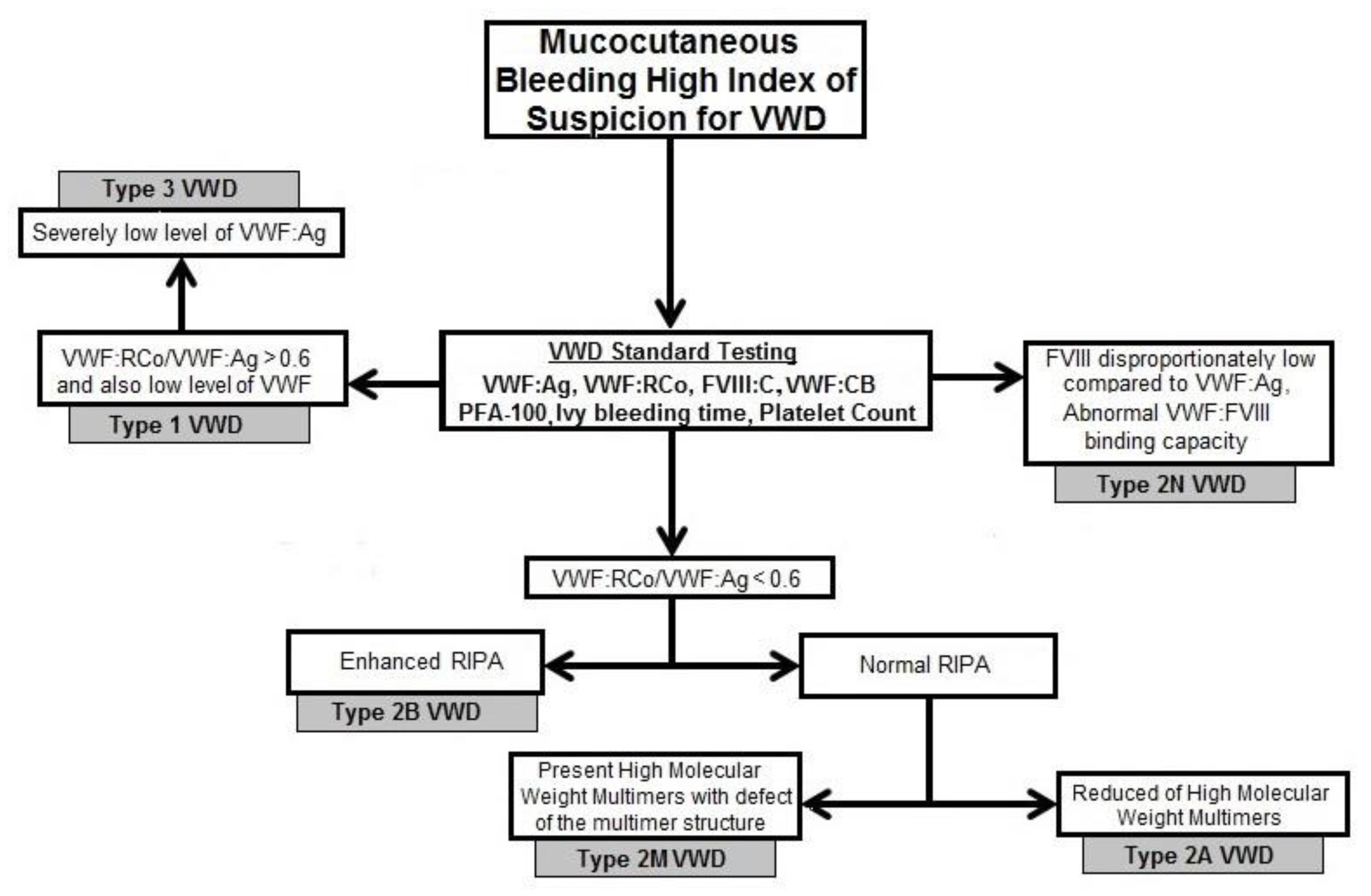
3. Results
4. Discussion
5. Implications for Clinical Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curnow, J.; Pasalic, L.; Favaloro, E.J. Treatment of von Willebrand disease. Semin. Thromb. Hemost 2016, 42, 133–146. [Google Scholar]
- Simurda, T.; Dobrotova, M.; Skornova, I.; Sokol, J.; Kubisz, P.; Stasko, J. Successful Use of a Highly Purified Plasma von Willebrand Factor Concentrate Containing Little FVIII for the Long-Term Prophylaxis of Severe (Type 3) von Willebrand’s Disease. Semin. Thromb. Hemost 2017, 43, 639–641. [Google Scholar]
- Favaloro, E.J. Appropriate laboratory assessment as a critical facet in the proper diagnosis and classification of von Willebrand disorder. Best Pract. Res. Clin. Haematol. 2001, 14, 299–319. [Google Scholar] [CrossRef]
- Favaloro, E.J. Von Willebrand disease: Local diagnosis and management of aglobally distributed bleeding disorder. Semin. Thromb. Hemost 2011, 37, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Schneppenheim, R.; Budde, U. Interactions of von Willebrand factor and ADAMTS13 in von Willebrand disease and thrombotic thrombocytopenic purpura. Hämostaseologie 2014, 34, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Škorňová, I.; Slavík, L.; Staško, J.; Kubisz, P.; Krčová, V.; Bártová, L.; Bradáčová, P.; Macichová, M.; Úlehlová, J.; Vážanová, A.; et al. Hemostáza; Laboratórne Metódy, ich Využitie a Interpretácia vo Vybraných Klinických Situáciach; Vydalo: P+M, s. r. o.; Laboratorní Metody, Jejich Využití a Interpretace ve Vybraných Klinických Situacích: Turany, Slovakia, 2020; pp. 85–94. ISBN 978-80-89694-78-5. [Google Scholar]
- Yee, A.; Kretz, C.A. Von Willebrand Factor: Form for Function. Semin. Thromb. Hemost. 2013, 40, 17–27. [Google Scholar] [PubMed]
- Haberichter, S.L.; Montgomery, R.M. Structure and function of von Willebrand factor. In Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 5th ed.; Colman, R.W., Ed.; Lippincott Williams&Wilkins: Philadelphia, PA, USA, 2006; p. 1827. [Google Scholar]
- Montgomery, R.R.; Haberichter, S.L. Von Willebrand Factor Structure and Function. In Von Willebrand Disease, 1st ed.; Federici, A.B., Ed.; Wiley-Blackwell: Oxford, UK, 2011; pp. 30–48. [Google Scholar]
- Nightingale, T.; Cutler, D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. J. Thromb. Haemost. 2013, 11, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Shiltagh, N.; Kirkpatrick, J.; Cabrita, L.D.; McKinnon, T.A.J.; Thalassinos, K.; Tuddenham, E.G.D.; Hansen, D.F. Solution structure of the major factor VIII binding region on von Willebrand factor. Blood 2014, 123, 4143–4151. [Google Scholar] [CrossRef] [Green Version]
- Favaloro, E.J. Towards personalised therapy for von Willebrand disease: A future role for recombinant products. Blood Transfus. 2016, 14, 262–276. [Google Scholar]
- Bowyer, A.E.; Goodfellow, K.J.; Nouadje, G.; Beaulieu, G.; Stufano, F.; Kitchen, S.; Makris, M. Performances of the Hydragel 5 von Willebrand multimers- a new within-day von Willebrand factor (VWF) multimer screening method Evaluation of a new commercial method for von Willebrand factor multimeric analysis. Res. Pract. Thromb. Haemost. 2017, 1, 494. [Google Scholar]
- Oliver, S.; Vanniasinkam, T.; Mohammed, S.; Vong, R.; Favaloro, E.J. Semi-automated von Willebrand factor multimer assay for von Willebrand disease: Further validation, benefits and limitations. Int. J. Lab. Hematol. 2019, 41, 762–771. [Google Scholar] [CrossRef]
- Seidel, H.; Westhofen, P.; Bautista, H.; Beaulieu, G.; Nouadje, G.; Kruppenbacher, J.P. Clinical evaluation of the sebia hydragel von Willebrand factor assay in comparison to electrophoresis and blotting based multimer analysis. Res. Pract. Thromb. Haemost. 2017, 1, 717–718. [Google Scholar]
- Vasse, M.; Francois, D.; Ligneel, T.; Bironien, R.; Nouadje, G. Interest of the new rapid test“ Hydragel 5 von willebrand multimers” for the analysis of von willebrand multimers. Int. J. Lab Hem. 2016, 38, 113. [Google Scholar]
- Pikta, M.; Zemtsovskaja, G.; Bautista, H.; Nouadje, G.; Szanto, T.; Viigimaa, M.; Banys, V. Preclinical evaluation of a semi-automated and rapid commercial electrophoresis assay for von Willebrand factor multimers. J. Clin. Lab. Anal. 2018, 32, e22416. [Google Scholar] [CrossRef] [PubMed]
- Vangenechten, I.; Gadisseur, A. Improving diagnosis of von Willebrand disease: Reference ranges for von Willebrand factor multimer distribution. Res. Pract. Thromb. Haemost. 2020, 4, 1024–1034. [Google Scholar] [CrossRef]
- Boender, J.; Atiq, F.; Cnossen, M.H.; van der Bom, J.G.; Fijnvandraat, K.; de Meris, J.; de Maat, M.P.M.; van Galen, K.P.M.; Gorkom, B.A.P.L.-V.; Meijer, K.; et al. Von Willebrand Factor Multimer Densitometric Analysis: Validation of the Clinical Accuracy and Clinical Implications in Von Willebrand Disease. HemaSphere 2021, 5, e542. [Google Scholar] [CrossRef]
- Goodfellow, K.J.; Bowyer, A.E.; Nouadje, G.; Bautista, H.; Beaulieu, G.; Kitchen, S.; Makris, M. Sebia Hydragel 5 von Willebrand multimers—A new and rapid von Willebrand factor (VWF) multimer screening method to aid subtyping of type 2 von Willebrand disease (VWD). Clin. Chem. Lab Med. 2017, 55, 721. [Google Scholar]
- Bowyer, A.E.; Goodfellow, K.J.; Seidel, H.; Westhofen, P.; Stufano, F.; Goodeve, A.; Kitchen, S.; Makris, M. Evaluation of a semi-automated von Willebrand factor multimer assay, the Hydragel 5 von Willebrand multimer, by two European Centers. Res. Pract. Thromb. Haemost. 2018, 2, 790–799. [Google Scholar] [CrossRef]
- Lemaitre, A.; Nouadje, G.; Beaulieu, G.; Bautista, H.; Desmet, S.; Gavard, C.; Eeckhoudt, S. Clinical evaluation of the Hydragel 5 von Willebrand multimers of Sebia. Clin. Chem. Lab Med. 2017, 55, 706. [Google Scholar]
- Crist, R.A.; Heikal, N.M.; Rodgers, G.M.; Grenache, D.G.; Smock, K.J. Evaluation of a new commercial method for von Willebrand factor multimeric analysis. Int. J. Lab. Hematol. 2018, 40, 586–591. [Google Scholar] [CrossRef]
- Zolkova, J.; Sokol, J.; Simurda, T.; Vadelova, L.; Sňahničanová, Z.; Loderer, D.; Dobrotova, M.; Ivankova, J.; Skornova, I.; Lasabova, Z.; et al. Genetic Background of von Willebrand Disease: History, Current State, and Future Perspectives. Semin. Thromb. Hemost. 2019, 46, 484–500. [Google Scholar] [CrossRef]
- Pikta, M.; Vasse, M.; Lejniece, S.; Smock, K.J.; Moser, K.A.; Bautista, H.; Nouadje, G.; Banys, V. Establishing Reference Intervals for von Willebrand Factor Multimers. Available online: https://abstracts.isth.org/abstract/establishing-reference-intervals-for-von-willebrand-factor-multimers/ (accessed on 20 November 2021).
- Kubisz, P.; Sokol, J.; Simurda, T.; Plamenova, I.; Dobrotova, M.; Holly, P.; Škorňová, I.; Staško, J. Diagnosis and management of von Willebrand disease in Slovakia. Ann. Blood 2018, 3, 9. [Google Scholar] [CrossRef]
- Favaloro, E.J. Rare forms of von Willebrand disease. Ann. Transl. Med. 2018, 6, 345. [Google Scholar] [CrossRef]
- Franchini, M.; Crestani, S.; Frattini, F.; Sissa, C.; Bonfanti, C. ABO blood group and von Willebrand factor: Biological implications. Clin. Chem. Lab. Med. 2014, 52, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.E. Low von Willebrand factor: Sometimes a risk factor and sometimes a disease. Hematol. Am. Soc. Hematol. Educ. Program. 2009, 2009, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, P.D.; Connell, N.T.; Ameer, B.; Di Paola, J.; Eikenboom, J.; Giraud, N.; Haberichter, S.; Jacobs-Pratt, V.; Konkle, B.; McLintock, C.; et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021, 5, 280–300. [Google Scholar] [CrossRef]
- Laffan, M.A.; Lester, W.; O’Donnell, J.; Will, A.; Tait, R.; Goodeve, A.; Millar, C.M.; Keeling, D.M. The diagnosis and management of von Willebrand disease: A United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br. J. Haematol. 2014, 167, 453–465. [Google Scholar] [CrossRef]
- Nichols, W.L.; Hultin, M.B.; James, A.H.; Manco-Johnson, M.J.; Montgomery, R.R.; Ortel, T.L.; Rick, M.E.; Sadler, J.E.; Weinstein, M.; Yawn, B.P. von Willebrand disease (VWD): Evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008, 14, 171–232. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Pasalic, L.; Curnow, J. Diagnosis and management of von Willebrand disease in Australia. Ann. Blood 2018, 3, 31. [Google Scholar] [CrossRef]
- Batlle, J.; Pérez-Rodríguez, A.; Corrales, I.; Borràs, N.; Rodríguez-Trillo, Á.; Lourés, E.; Cid, A.R.; Bonanad, S.; Cabrera, N.; Moret, A.; et al. Diagnosis and management of von willebrand disease in Spain. Ann. Blood 2018, 3, 5. [Google Scholar] [CrossRef]
- Flood, V.H.; The Zimmerman Program Investigators; Abshire, T.C.; Christopherson, P.A.; Friedman, K.D.; Gill, J.C.; Montgomery, R.R.; Haberichter, S.L. Von Willebrand disease in the United States: Perspective from the Zimmerman program. Ann. Blood 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Pruthi, R.K. A Practical Approach to Genetic Testing for von Willebrand Disease. Mayo Clin. Proc. 2006, 81, 679–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneppenheim, R. The evolving classification of von Willebrand disease. Blood Coagul. Fibrinolysis 2005, 16, S3–S10. [Google Scholar] [CrossRef]
- Casonato, A.; Daidone, V.; Galletta, E.; Bertomoro, A. Type 2B von Willebrand disease with or without large multimers: A distinction of the two sides of the disorder is long overdue. PLoS ONE 2017, 12, e0179566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, S.; Abuzenadah, A.M.; Blagg, J.L.; Hinks, J.; Nesbitt, I.M.; Goodeve, A.; Gursel, T.; Ingerslev, J.; Peake, I.R.; Daly, M.E. Two novel type 2N von Willebrand disease—Causing mutations that result in defective factor VIII binding, multimerization, and secretion of von Willebrand factor. Blood 2000, 95, 2000–2007. [Google Scholar] [CrossRef]


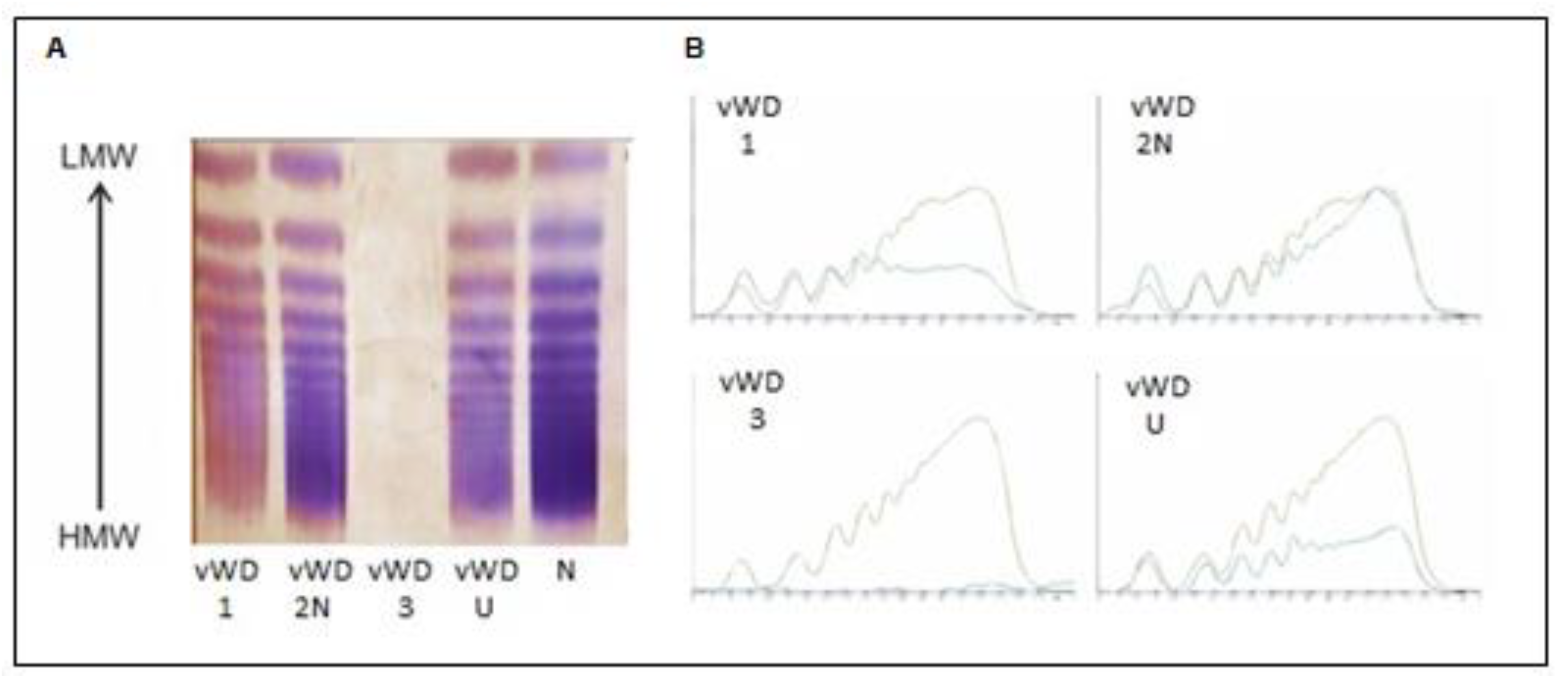
| Sample No. | VWF—Ac (%) NR: 50–140% | VWF—Ag (%) NR: 60–150% | VWF—Ac/VWF—Ag | FVIII—C (%) NR: 60–150% | CBA (%) NR: 50–150% | CBA/VWF—Ag | Multimers (%) LMW (NR: 12–24%) IMW (NR: 25–35%) HMW (NR: 41–70%) | VWD Type |
|---|---|---|---|---|---|---|---|---|
| 1 | 34 | 46 | 0.74 | 101 | 105 | 2.28 | LMW 28% IMW 25% HMW 26% | VWD type 1 |
| 2 | 129 | 108 | 1.19 | 39 | 73 | 0.68 | LMW 20% IMW 25% HMW 45% | VWD type 2N |
| 3 | 7 | 0.5 | uncalculable | 5 | 6 | uncalculable | LMW 3.3% IMW 1% HMW 3% | VWD type 3 |
| 4 | 26 | 38 | 0.68 | 92 | 71 | 1.87 | LMW 17.4% IMW 17.5% HMW 27% | VWD unclassified |
| Sample No. | VWF—Ac % NR: 50–140 | VWF—Ag % NR: 60–150 | VWF—Ac/ VWF—Ag | FVIII % NR: 60–150 | CBA % NR: 50–150 | CBA/VWF—Ag | Multimers (%) LMW (NR: 12–24%) IMW (NR: 25–35%) HMW (NR: 41–70%) | VWD |
|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 32 | 0.75 | 71 | 31 | 0.97 | LMW 10% IMW 15% HMW 24% | VWD type 1 |
| 2 | 0.5 | 3 | 0.17 | 9 | <0.5 | uncalculable | LMW 4% IMW 2% HMW 0% | VWD type 3 |
| 3 | 7 | 0.5 | uncalculable | 5 | 6 | uncalculable | LMW 3.3% IMW 1% HMW 3% | VWD type 3 |
| 4 | 26 | 38 | 0.68 | 92 | 71 | 1.87 | LMW 17.4% IMW 17.5% HMW 27% | VWD unclassified |
| 5 | 34 | 46 | 0.74 | 101 | 105 | 2.28 | LMW 28% IMW 25% HMW 26% | VWD type 1 |
| 6 | 129 | 108 | 1.19 | 39 | 73 | 0.68 | LMW 20% IMW 25% HMW 45% | VWD type 2N |
| 7 | 18 | 32 | 0.56 | 67 | 15 | 0.47 | LMW 13% IMW 10% HMW 14% | VWD type 1/2A |
| 8 | 29 | 41 | 0.71 | 62 | 11 | 0.27 | LMW 12% IMW 7% HMW 8% | VWD type 1/2A |
| 9 | 45 | 54 | 0.83 | 80 | 65 | 1.20 | LMW 7% IMW 4% HMW 5% | VWD unclassified |
| 10 | 50 | 55 | 0.91 | 59 | 59 | 1.07 | LMW 6% IMW 6.8% HMW 6% | VWD unclassified |
| 11 | 86 | 110 | 0.78 | 150 | 71 | 0.65 | LMW 10% IMW 17% HMW 29% | VWD unclassified |
| 12 | 58 | 56 | 1.04 | 69 | 71 | 1.27 | LMW 10% IMW 17% HMW 29% | VWD type 1 |
| 13 | 25 | 38 | 0.66 | 195 | 31 | 0.82 | LMW 34% IMW 32% HMW 45% | VWD type 1 |
| 14 | 44 | 50 | 0.88 | 58 | 59 | 1.18 | LMW 12% IMW 17% HMW 35% | VWD type 1 |
| 16 | 69 | 64 | 1.08 | 120 | 76 | 1.19 | LMW 13% IMW 19% HMW 30% | VWD type 1 |
| 17 | 64 | 83 | 0.77 | 90 | 72 | 0.87 | LMW 25% IMW 24% HMW 36% | VWD type 1 |
| 18 | 65 | 90 | 0.72 | 90 | 82 | 0.91 | LMW 12% IMW 16% HMW 26% | VWD type 1 |
| 20 | 47 | 116 | 0.41 | 104 | 73 | 0.63 | LMW 33% IMW 40% HMW 41% | VWD unclassified |
| 24 | 120 | 124 | 0.97 | 100 | 97 | 0.78 | LMW 35% IMW 43% HMW 65% | VWD unclassified |
| 25 | 47 | 55 | 0.85 | 44 | 81 | 1.47 | LMW 15% IMW 19% HMW 38% | VWD type 1 |
| 26 | 1.1 | 4.4 | 0.25 | 16 | <0.5 | uncalculable | LMW 3.2% IMW 1.5% HMW 0% | VWD type 3 |
| 27 | 2 | 0.5 | uncalculable | 3.5 | 7 | uncalculable | LMW 1.7% IMW 0.1% HMW 1.9% | VWD type 1 |
| 28 | 63 | 68 | 0.93 | 91 | 73 | 1.07 | LMW 25% IMW 8% HMW 16% | VWD type 1 |
| 29 | 67 | 65 | 1.03 | 122 | 63 | 0.97 | LMW 23% IMW 20% HMW 37% | VWD type 1 |
| 30 | 70 | 80 | 0.88 | 50 | 72 | 0.90 | LMW 17% IMW 12% HMW 21% | VWD type 1 |
| 38 | 55 | 58 | 0.95 | 92 | 52 | 0.90 | LMW 37% IMW 28% HMW 35% | VWD unclassified |
| 39 | 27 | 33 | 0.82 | 60 | 10 | 0.30 | LMW 22% IMW 15% HMW 23% | VWD type 1 |
| 42 | 22 | 33 | 0.67 | 29 | 20 | 0.61 | LMW 9% IMW 1% HMW 6% | VWD type 1 |
| 43 | 15 | 25 | 0.60 | 45 | 30 | 1.20 | LMW 11% IMW 3% HMW 13% | VWD type 1 |
| 44 | 39 | 55 | 0.71 | 136 | 72 | 1.31 | LMW 25% IMW 30% HMW 42% | VWD type 1 |
| 48 | 60 | 66 | 0.91 | 90 | 70 | 1.06 | LMW 17% IMW 17% HMW 30% | VWD type 1 |
| 49 | 0.8 | 0.5 | 1.60 | 0.9 | <0.5 | uncalculable | LMW 9% IMW 1% HMW 7% | VWD type 1 |
| 50 | 7.6 | 15 | 0.51 | 34 | 6 | 0.40 | LMW 14% IMW 2.5% HMW 11% | VWD type 2A |
| 51 | 44 | 50 | 0.88 | 88 | 47 | 0.94 | LMW 12% IMW 9% HMW 23% | VWD type 1 |
| 52 | 15 | 11 | 1.36 | 49 | 6.4 | 0.58 | LMW 17.3% IMW 1.1% HMW 5.8% | VWD type 1/2A |
| 53 | 26 | 46 | 0.57 | 49 | 28 | 0.61 | LMW 34% IMW 14% HMW 8% | VWD type 2A |
| 54 | 10 | 32 | 0.31 | 101 | 11 | 0.34 | LMW 39% IMW 41% HMW 61% | VWD unclassified |
| 55 | 36 | 38 | 0.95 | 59 | 35 | 0.92 | LMW 17% IMW 8% HMW 17% | VWD type 1 |
| 56 | 23 | 32 | 0.72 | 63 | 37 | 1.16 | LMW 15% IMW 12% HMW 31% | VWD type 1 |
| 57 | 48 | 65 | 0.74 | 82 | 73 | 1.12 | LMW 16% IMW 14% HMW 33% | VWD unclassified |
| 58 | 72 | 77 | 0.94 | 124 | 73 | 0.95 | LMW 19% IMW 15% HMW 34% | VWD unclassified |
| 59 | 19 | 6 | uncalculable | 12 | 27 | uncalculable | LMW 14% IMW 4% HMW 5% | VWD type 3 |
| 60 | 34 | 36 | 0.94 | 69 | 35 | 0.97 | LMW 11% IMW 11% HMW 27% | VWD type 1 |
| 61 | 16 | 25 | 0.64 | 21 | 20 | 0.80 | LMW 22% IMW 14% HMW 19% | VWD type 1 |
| 62 | 56 | 54 | 1.04 | 100 | 81 | 1.50 | LMW 20% IMW 15% HMW 37% | VWD type 1 |
| 63 | 36 | 30 | 1.20 | 123 | 41 | 1.37 | LMW 2% IMW 3% HMW 35% | VWD type 1 |
| 64 | 39 | 41 | 0.95 | 80 | 57 | 1.39 | LMW 15% IMW 8% HMW 29% | VWD type 1 |
| 65 | 30 | 34 | 0.88 | 59 | 52 | 1.53 | LMW 13% IMW 10% HMW 29% | VWD type 1 |
| 66 | 22 | 25 | 0.88 | 72 | 60 | 2.40 | LMW 12% IMW 9% HMW 24% | VWD type 1 |
| 67 | 44 | 45 | 0.98 | 111 | 35 | 0.78 | LMW 11% IMW 11% HMW 22% | VWD type 1 |
| 68 | 35 | 30 | 1.17 | 81 | 59 | 1.97 | LMW 12% IMW 9% HMW 13% | VWD type 1 |
| 69 | 61 | 58 | 1.05 | 99 | 58 | 1.00 | LMW 14% IMW 15% HMW 24% | VWD type 1 |
| 70 | 15 | 28 | 0.54 | 70 | 14 | 0.50 | LMW 10% IMW 7% HMW 13% | VWD type 1 |
| 71 | 15 | 24 | 0.63 | 58 | 15 | 0.63 | LMW 8% IMW 7% HMW 15% | VWD type 1 |
| 72 | 45 | 63 | 0.71 | 72 | 65 | 1.03 | LMW 18% IMW 14% HMW 21% | VWD type 1 |
| 73 | 42 | 48 | 0.88 | 64 | 57 | 1.19 | LMW 17% IMW 11% HMW 19% | VWD type 1 |
| 74 | 62 | 53 | 1.17 | 97 | 78 | 1.47 | LMW 10% IMW 11% HMW 24% | VWD type 1 |
| 75 | 48 | 69 | 0.70 | 111 | 59 | 0.86 | LMW 12% IMW 10% HMW 30% | VWD type 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skornova, I.; Simurda, T.; Stasko, J.; Zolkova, J.; Sokol, J.; Holly, P.; Dobrotova, M.; Plamenova, I.; Hudecek, J.; Brunclikova, M.; et al. Multimer Analysis of Von Willebrand Factor in Von Willebrand Disease with a Hydrasys Semi-Automatic Analyzer—Single-Center Experience. Diagnostics 2021, 11, 2153. https://doi.org/10.3390/diagnostics11112153
Skornova I, Simurda T, Stasko J, Zolkova J, Sokol J, Holly P, Dobrotova M, Plamenova I, Hudecek J, Brunclikova M, et al. Multimer Analysis of Von Willebrand Factor in Von Willebrand Disease with a Hydrasys Semi-Automatic Analyzer—Single-Center Experience. Diagnostics. 2021; 11(11):2153. https://doi.org/10.3390/diagnostics11112153
Chicago/Turabian StyleSkornova, Ingrid, Tomas Simurda, Jan Stasko, Jana Zolkova, Juraj Sokol, Pavol Holly, Miroslava Dobrotova, Ivana Plamenova, Jan Hudecek, Monika Brunclikova, and et al. 2021. "Multimer Analysis of Von Willebrand Factor in Von Willebrand Disease with a Hydrasys Semi-Automatic Analyzer—Single-Center Experience" Diagnostics 11, no. 11: 2153. https://doi.org/10.3390/diagnostics11112153
APA StyleSkornova, I., Simurda, T., Stasko, J., Zolkova, J., Sokol, J., Holly, P., Dobrotova, M., Plamenova, I., Hudecek, J., Brunclikova, M., Stryckova, A., & Kubisz, P. (2021). Multimer Analysis of Von Willebrand Factor in Von Willebrand Disease with a Hydrasys Semi-Automatic Analyzer—Single-Center Experience. Diagnostics, 11(11), 2153. https://doi.org/10.3390/diagnostics11112153







