Role of Endoscopic Ultrasound in Liver Disease: Where Do We Stand?
Abstract
:1. Introduction
2. EUS in Diffuse Liver Lesions
2.1. EUS Elastography
2.2. EUS-Guided Liver Biopsy (EUS-LB)
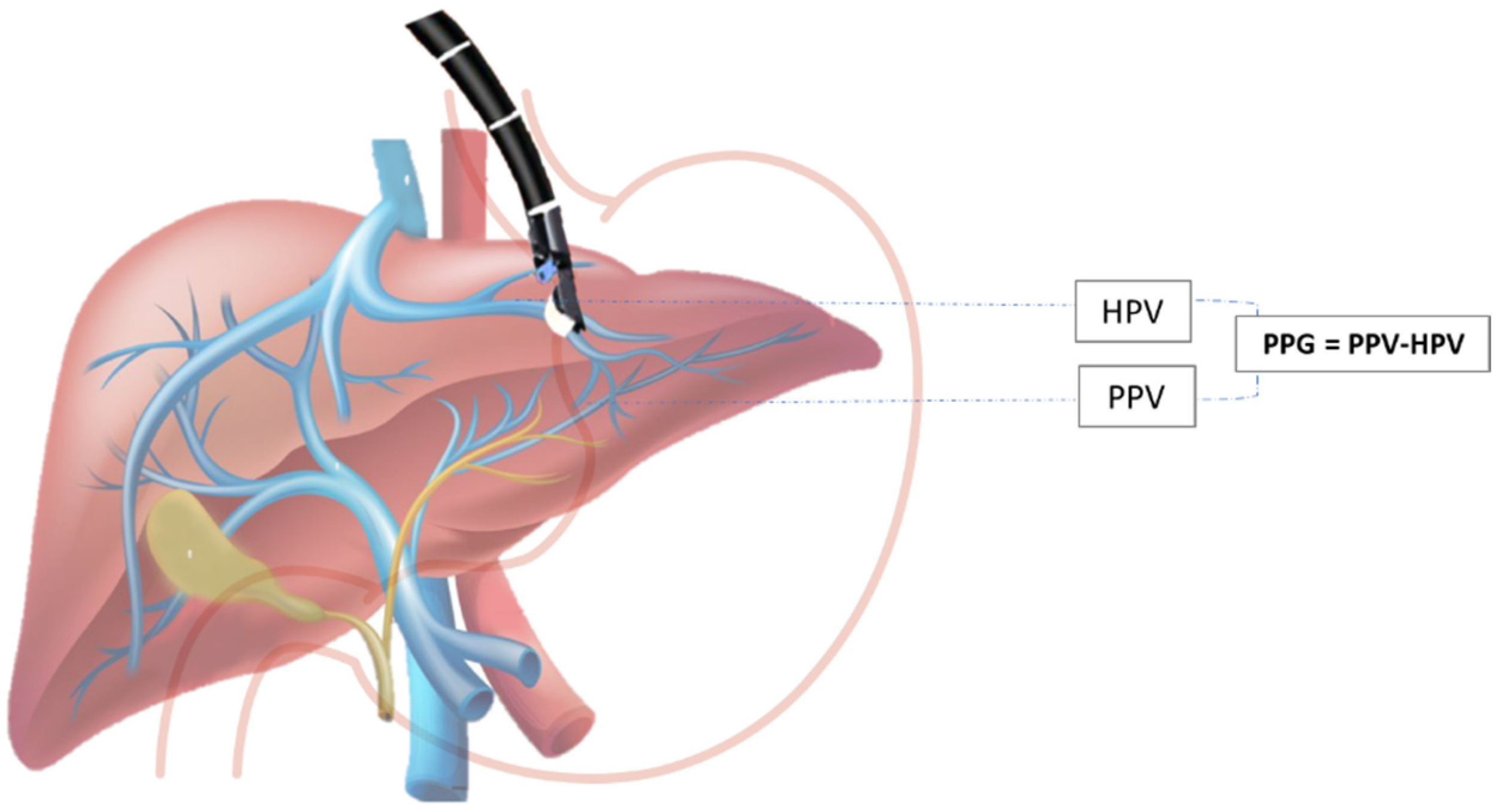
2.3. EUS-Guided Portal Hypertension Measurement
2.4. Detection of Varices and Prediction of Variceal Bleeding
2.5. EUS-Guided Therapy for PH
3. EUS in Diagnostic Evaluation of Focal Liver Lesions
3.1. EUS FNA/FNB
3.2. EUS Elastography
3.3. Contrast-Enhancement EUS (CE–EUS)
4. Staging of Pancreatobiliary Malignancies
5. EUS-Guided Treatment of Hepatic Lesions
5.1. Treatment of Cystic Liver Lesions
5.2. Drainage of Liver Abscesses
5.3. Treatment of Solid Liver Lesions
5.3.1. FNI Therapy
Ethanol Injection Therapy
EUS-Guided Portal Injection Chemotherapy (EPIC)
5.3.2. Thermoablative Therapies
Clinical Application of RFA Has So Far Been Noted Only in Case-Reports with Fair Success
5.3.3. EUS-Guided Brachytherapy and Fiducial Placement
5.3.4. Photodynamic Therapy
6. EUS in Primary Sclerosing Cholangitis and Undetermined Biliary Strictures
7. Limitations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 392, 1789–1858. [Google Scholar] [CrossRef]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, T.R.; Bazarbashi, A.N.; Njei, B.; Ryou, M.; Aslanian, H.R.; Muniraj, T. Endoscopic Ultrasound-Guided, Percutaneous, and Transjugular Liver Biopsy: A Comparative Systematic Review and Meta-Analysis. Clin. Endosc. 2020, 53, 583–593. [Google Scholar] [CrossRef]
- Rimbaş, M.; Di Maurizio, L.; Rizzatti, G.; Gasbarrini, A.; Costamagna, G.; Larghi, A. Endoscopic Ultrasound for the Hepatologist: A Comprehensive Review. Semin. Liver Dis. 2018, 38, 145–159. [Google Scholar] [CrossRef]
- Eddowes, P.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikolasevic, I.; Domislovic, V.; Klapan, M.; Juric, T.; Lukic, A.; Krznaric-Zrnic, I.; Fuckar-Cupic, D.; Stimac, D.; Kanizaj, T.F.; Krznaric, Z.; et al. Accuracy of Controlled Attenuation Parameter and Liver Stiffness Measurement in Patients with Non-alcoholic Fatty Liver Disease. Ultrasound Med. Biol. 2021, 47, 428–437. [Google Scholar] [CrossRef]
- Campos, S.; Poley, J.-W.; Van Driel, L.; Bruno, M.J. The role of EUS in diagnosis and treatment of liver disorders. Endosc. Int. Open 2019, 7, E1262–E1275. [Google Scholar] [CrossRef] [Green Version]
- Fung, B.M.; Abadir, A.P.; Eskandari, A.; Levy, M.J.; Tabibian, J.H. Endoscopic ultrasound in chronic liver disease. World J. Hepatol. 2020, 12, 262–276. [Google Scholar] [CrossRef]
- Mahfouz, M.; Amin, S.; Carrion, A.F. The Evolving Role of Advanced Endoscopic Techniques in Hepatology. Gastroenterol. Hepatol. 2021, 17, 67–72. [Google Scholar]
- Song, J.E.; Lee, D.W.; Kim, E.Y. Endoscopic Ultrasound Real-Time Elastography in Liver Disease. Clin. Endosc. 2018, 51, 118–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, D.E.; Ma, M.; Kadosh, D.; Menon, A.; Chin, K.; Swaminath, A. Endo-hepatology: An emerging field. World J. Gastrointest. Endosc. 2021, 13, 296–301. [Google Scholar] [CrossRef]
- Brattain, L.J.; Telfer, B.A.; Dhyani, M.; Grajo, J.R.; Samir, A.E. Objective Liver Fibrosis Estimation from Shear Wave Elas-tography. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society Annual International Conference, Honolulu, HI, USA, 17–21 July 2018. [Google Scholar]
- Schulman, A.R.; Lin, M.V.; Rutherford, A.; Chan, W.W.; Ryou, M. A Prospective Blinded Study of Endoscopic Ultrasound Elastography in Liver Disease: Towards a Virtual Biopsy. Clin. Endosc. 2018, 51, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Sandulescu, L.; Padureanu, V.; Dumitrescu, C.; Braia, N.; Streba, C.T.; Gheonea, D.; Cazacu, S.; Ciurea, T.; Rogoveanu, I.; Saftoiu, A. A Pilot Study of Real Time Elastography in the Differentiation of Focal Liver Lesions. Curr. Health Sci. J. 2012, 38, 32–35. [Google Scholar]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D. American Association for the Study of Liver Dis-eases Liver biopsy. Hepatology 2008, 49, 1017–1044. [Google Scholar] [CrossRef]
- Kalambokis, G.; Manousou, P.; Vibhakorn, S.; Marelli, L.; Cholongitas, E.; Senzolo, M.; Patch, D.; Burroughs, A.K. Transjugular liver biopsy–Indications, adequacy, quality of specimens, and complications–A systematic review. J. Hepatol. 2007, 47, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.P.; Fong, A.; Shaheen, A.A.M. Utilization rates, complications and costs of percutaneous liver biopsy: A population-based study including 4275 biopsies. Liver Int. 2008, 28, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Mammen, T.; Keshava, S.N.; Eapen, C.; Raghuram, L.; Moses, V.; Gopi, K.; Babu, N.S.; Ramachandran, J.; Kurien, G. Transjugular Liver Biopsy: A Retrospective Analysis of 601 Cases. J. Vasc. Interv. Radiol. 2008, 19, 351–358. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Card, T.R. Reduced Mortality Rates Following Elective Percutaneous Liver Biopsies. Gastroenterology 2010, 139, 1230–1237. [Google Scholar] [CrossRef]
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.-Z.; Reddy, K.; Schiff, E.R. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 2002, 97, 2614–2618. [Google Scholar] [CrossRef]
- Mathew, A. EUS-Guided Routine Liver Biopsy in Selected Patients. Am. J. Gastroenterol. 2007, 102, 2354–2355. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, F.C.; Clayton, A.C.; Zhang, L.; Clain, J.E.; Gores, G.J.; Rajan, E.; Smyrk, T.C.; Topazian, M.D.; Wang, K.K.; Wiersema, M.J.; et al. Adequacy of Endoscopic Ultrasound Core Needle Biopsy Specimen of Nonmalignant Hepatic Parenchymal Disease. Clin. Gastroenterol. Hepatol. 2008, 6, 1437–1440. [Google Scholar] [CrossRef]
- DeWitt, J.; McGreevy, K.; Cummings, O.; Sherman, S.; LeBlanc, J.K.; McHenry, L.; Al-Haddad, M.; Chalasani, N. Initial experience with EUS-guided Tru-cut biopsy of benign liver disease. Gastrointest. Endosc. 2009, 69, 535–542. [Google Scholar] [CrossRef]
- Baron, T.; Parekh, P.; Majithia, R.; Diehl, D.L.; Baron, T.H. Endoscopic ultrasound-guided liver biopsy. Endosc. Ultrasound 2015, 4, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Stavropoulos, S.N.; Im, G.; Jlayer, Z.; Harris, M.D.; Pitea, T.C.; Turi, G.K.; Malet, P.F.; Friedel, D.M.; Grendell, J.H. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest. Endosc. 2012, 75, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Vilmann, P.; Krasnik, M.; Larsen, S.S.; Jacobsen, G.K.; Clementsen, P.F. Transesophageal Endoscopic Ultrasound-Guided Fine-Needle Aspiration (EUS-FNA) and Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA) Biopsy: A Combined Approach in the Evaluation of Mediastinal Lesions. Endoscopy 2005, 37, 833–839. [Google Scholar] [CrossRef]
- Johnson, K.D.; Laoveeravat, P.; Yee, E.U.; Perisetti, A.; Thandassery, R.B.; Tharian, B. Endoscopic ultrasound guided liver biopsy: Recent evidence. World J. Gastrointest. Endosc. 2020, 12, 83–97. [Google Scholar] [CrossRef]
- Diehl, D.L. Endoscopic Ultrasound–guided Liver Biopsy. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Gor, N.; Salem, S.B.; Jakate, S.; Patel, R.; Shah, N.; Patil, A. Histological adequacy of EUS-guided liver biopsy when using a 19-gauge non–Tru-Cut FNA needle. Gastrointest. Endosc. 2014, 79, 170–172. [Google Scholar] [CrossRef]
- Pineda, J.J.; Diehl, D.L.; Miao, C.L.; Johal, A.S.; Khara, H.S.; Bhanushali, A.; Chen, E.Z. EUS-guided liver biopsy provides diagnostic samples comparable with those via the percutaneous or transjugular route. Gastrointest. Endosc. 2016, 83, 360–365. [Google Scholar] [CrossRef]
- Sey, M.S.L.; Al-Haddad, M.; Imperiale, T.F.; McGreevy, K.; Lin, J.; DeWitt, J.M. EUS-guided liver biopsy for parenchymal disease: A comparison of diagnostic yield between two core biopsy needles. Gastrointest. Endosc. 2016, 83, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Phan, J.; Jimenez, M.A.; Grotts, J.F.; Walters, L.; Hathaway, K.A.; Patel, K.R.; Lankarani, A.; Herman, M.; Holloman, D.A.; et al. Endoscopic Ultrasound Liver Biopsies Accurately Predict the Presence of Fibrosis in Patients with Fatty liver. Clin. Gastroenterol. Hepatol. 2017, 15, 1477–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.D.; Sasatomi, E.; Baron, T.H. Endoscopic Ultrasound–guided Parenchymal Liver Biopsy: Single Center Experience of a New Dedicated Core Needle. Clin. Gastroenterol. Hepatol. 2017, 15, 784–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mok, S.R.; Diehl, D.L.; Johal, A.S.; Khara, H.S.; Confer, B.D.; Mudireddy, P.R.; Kirchner, H.L.; Chen, Z.-M.E. A prospective pilot comparison of wet and dry heparinized suction for EUS-guided liver biopsy (with videos). Gastrointest. Endosc. 2018, 88, 919–925. [Google Scholar] [CrossRef]
- Nieto, J.; Khaleel, H.; Challita, Y.; Jimenez, M.; Baron, T.H.; Walters, L.; Hathaway, K.; Patel, K.; Lankarani, A.; Herman, M.; et al. EUS-guided fine-needle core liver biopsy sampling using a novel 19-gauge needle with modified 1-pass, 1 actuation wet suction technique. Gastrointest. Endosc. 2018, 87, 469–475. [Google Scholar] [CrossRef]
- Ching-Companioni, R.A.; Diehl, D.L.; Johal, A.S.; Confer, B.D.; Khara, H.S. 19 G aspiration needle versus 19 G core biopsy needle for endoscopic ultrasound-guided liver biopsy: A prospective randomized trial. Endoscopy 2019, 51, 1059–1065. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.K.; Kadkhodayan, K.; Idrisov, E.; Ali, S.; Rafiq, E.; Shor, D.B.-A.; Abdel-Jalil, A.; Navaneethan, U.; Bang, J.; Varadarajulu, S.; et al. Endoscopic ultrasound-guided liver biopsy using a 22-G fine needle biopsy needle: A prospective study. Endoscopy 2019, 51, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazerbachi, F.; Vargas, E.J.; Matar, R.; Storm, A.C.; Mounajjed, T.M.; Topazian, M.D.; Levy, M.J.; Chandrasekhara, V.; Abu Dayyeh, B.K. EUS-guided core liver biopsy sampling using a 22-gauge fork-tip needle: A prospective blinded trial for histologic and lipidomic evaluation in nonalcoholic fatty liver disease. Gastrointest. Endosc. 2019, 90, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.R.S.; Diehl, D.L.; Johal, A.S.; Khara, H.S.; Confer, B.D.; Mudireddy, P.R.; Kovach, A.H.; Diehl, M.M.; Kirchner, H.L.; Chen, Z.-M.E. Endoscopic ultrasound-guided biopsy in chronic liver disease: A randomized comparison of 19-G FNA and 22-G FNB needles. Endosc. Int. Open 2019, 7, E62–E71. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; Butt, W.; Khara, H.S.; Johal, A.S.; West, S.F.; Chen, Z.-M.E.; Berger, A.L.; Diehl, D.L. Bi-lobar liver biopsy via EUS enhances the assessment of disease severity in patients with non-alcoholic steatohepatitis. Hepatol. Int. 2019, 13, 323–329. [Google Scholar] [CrossRef]
- Shuja, A.; Alkhasawneh, A.; Fialho, A.; Shukri, A.; Harris, C.; Smotherman, C.; Malespin, M.; de Melo, S.W. Comparison of EUS-guided versus percutaneous and transjugular approaches for the performance of liver biopsies. Dig. Liver Dis. 2019, 51, 826–830. [Google Scholar] [CrossRef]
- Aggarwal, S.N.; Magdaleno, T.; Klocksieben, F.; MacFarlan, J.E.; Goonewardene, S.; Zator, Z.; Shah, S.; Shah, H.N. A prospective, head-to-head comparison of 2 EUS-guided liver biopsy needles in vivo. Gastrointest. Endosc. 2021, 93, 1133–1138. [Google Scholar] [CrossRef]
- Nieto, J.; Dawod, E.; Deshmukh, A.; Penn, E.; Adler, D.; Saab, S. EUS-guided fine-needle core liver biopsy with a modified one-pass, one-actuation wet suction technique comparing two types of EUS core needles. Endosc. Int. Open 2020, 8, E938–E943. [Google Scholar] [CrossRef]
- Hashimoto, R.; Lee, D.P.; Samarasena, J.B.; Chandan, V.S.; Guo, W.; Lee, J.G.; Chang, K.J. Comparison of Two Specialized Histology Needles for Endoscopic Ultrasound (EUS)-Guided Liver Biopsy: A Pilot Study. Dig. Dis. Sci. 2021, 66, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Panchal, S.; Rao, D.S.; Gan, Y.; Al-Juboori, A.; Samiullah, S.; Ibdah, J.A.; Hammoud, G.M. The efficacy and safety of endoscopic ultrasound-guided liver biopsy versus percutaneous liver biopsy in patients with chronic liver disease: A retrospective single-center study. J. Ultrasound 2020, 23, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K.; Saxena, R.; Rush, N.; Patel, S.K.; Dasari, C.S.; Mneimneh, W.; Quickery, A.; Rahal, M.A.; Temnykh, L.; DeWitt, J.; et al. A Comparative Study of 22G versus 19G Needles for EUS-Guided Biopsies for Parenchymal Liver Disease: Are Thinner Needles Better? Dig. Dis. Sci. 2021, 66, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Ward, T.J.; Guirguis, S.; Krall, K.; Contreras, F.; Jhala, N.; Navaneethan, U.; Hawes, R.H.; Varadarajulu, S. Radiology-guided percutaneous approach is superior to EUS for performing liver biopsies. Gut 2021. [Google Scholar] [CrossRef]
- Mohan, B.P.; Shakhatreh, M.; Garg, R.; Ponnada, S.; Adler, D.G. Efficacy and safety of EUS-guided liver biopsy: A systematic review and meta-analysis. Gastrointest. Endosc. 2019, 89, 238–246.e3. [Google Scholar] [CrossRef]
- Confer, B.D.; Walker, J.T.; Khurana, S.; Unzueta, A.; Khara, H.S.; Johal, A.S.; Diehl, D.L. EUS-guided liver biopsy: The type of needle matters. Gastrointest. Endosc. 2019, 90, 321–322. [Google Scholar] [CrossRef] [Green Version]
- Schulman, A.R.; Thompson, C.C.; Odze, R.; Chan, W.W.; Ryou, M. Optimizing EUS-guided liver biopsy sampling: Comprehensive assessment of needle types and tissue acquisition techniques. Gastrointest. Endosc. 2017, 85, 419–426. [Google Scholar] [CrossRef]
- Obaitan, I.; Al-Haddad, M.A. How Can We Optimize Tools and Techniques for Endoscopic Ultrasound–Guided Liver Biopsy? Clin. Gastroenterol. Hepatol. 2020, 18, 1025–1027. [Google Scholar] [CrossRef]
- Baran, B.; Kale, S.; Patil, P.; Kannadath, B.; Ramireddy, S.; Badillo, R.; DaVee, R.T.; Thosani, N. Endoscopic ultrasound-guided parenchymal liver biopsy: A systematic review and meta-analysis. Surg. Endosc. 2021, 35, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, N.; Ou, G.; Lam, E.; Enns, R.; Telford, J. When trainees reach competency in performing endoscopic ultrasound: A systematic review. Endosc. Int. Open 2017, 5, E239–E243. [Google Scholar] [CrossRef] [Green Version]
- Chetwood, J.D.; Mudaliar, S.; Staudenmann, D.; Shin, J.-S.; Liu, K.; Majumdar, A.; Kaffes, A.; Strasser, S.; McCaughan, G.W.; Saxena, P. Emerging role of endoscopic ultrasound-guided liver biopsy. Gut 2020, 70, 1600–1601. [Google Scholar] [CrossRef]
- Suk, K.T. Hepatic venous pressure gradient: Clinical use in chronic liver disease. Clin. Mol. Hepatol. 2014, 20, 6–14. [Google Scholar] [CrossRef]
- Escorsell, À.; Bru, C.; Gilabert, R.; Moitinho, E.; Garcia-Pagan, J.C.; Bosch, J. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology 1999, 30, 1393–1397. [Google Scholar] [CrossRef]
- Fujii-Lau, L.; Leise, M.; Kamath, P.; Gleeson, F.; Levy, M. Endoscopic ultrasound-guided portal-systemic pressure gradient measurement. Endoscopy 2014, 46, E654–E656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.Y.; Samarasena, J.B.; Tsujino, T.; Lee, J.; Hu, K.-Q.; McLaren, C.E.; Chen, W.-P.; Chang, K.J. EUS-guided portal pressure gradient measurement with a simple novel device: A human pilot study. Gastrointest. Endosc. 2017, 85, 996–1001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Peng, C.; Zhang, S.; Huang, S.; Shen, S.; Xu, G.; Zhang, F.; Xiao, J.; Zhang, M.; Zhuge, Y.; et al. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest. Endosc. 2021, 93, 565–572. [Google Scholar] [CrossRef]
- Bazarbashi, A.N.; Ryou, M. Portal pressure measurement: Have we come full circle? Gastrointest. Endosc. 2021, 93, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Braden, B.; Gupta, V.; Dietrich, C. Therapeutic EUS: New tools, new devices, new applications. Endosc. Ultrasound 2019, 8, 370–381. [Google Scholar] [CrossRef]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- El-Saadany, M.; Jalil, S.; Irisawa, A.; Shibukawa, G.; Ohira, H.; Bhutani, M.S. EUS for portal hypertension: A comprehensive and critical appraisal of clinical and experimental indications. Endoscopy 2008, 40, 690–696. [Google Scholar] [CrossRef]
- Irisawa, A.; Obara, K.; Sato, Y.; Saito, A.; Takiguchi, F.; Shishido, H.; Sakamoto, H.; Kasukawa, R. EUS analysis of collateral veins inside and outside the esophageal wall in portal hypertension. Gastrointest. Endosc. 1999, 50, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Saraireh, H.A.; Bilal, M.; Singh, S. Role of endoscopic ultrasound in liver disease: Where do we stand in 2017? World J. Hepatol. 2017, 9, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, A.; Saito, A.; Obara, K.; Shibukawa, G.; Takagi, T.; Shishido, H.; Sakamoto, H.; Sato, Y.; Kasukawa, R. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: Severe periesophageal collateral veins and large perforating veins. Gastrointest. Endosc. 2001, 53, 77–84. [Google Scholar] [CrossRef]
- Mašalaitė, L.; Valantinas, J.; Stanaitis, J. Endoscopic ultrasound findings predict the recurrence of esophageal varices after endoscopic band ligation: A prospective cohort study. Scand. J. Gastroenterol. 2015, 50, 1322–1330. [Google Scholar] [CrossRef]
- Sato, T.; Yamazaki, K.; Toyota, J.; Karino, Y.; Ohmura, T.; Akaike, J. Endoscopic ultrasonographic evaluation of hemodynamics related to variceal relapse in esophageal variceal patients. Hepatol. Res. 2009, 39, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Kim, H.S.; Kim, S.G.; Yoo, J.-J.; Jang, J.Y.; Lee, S.H.; Kim, H.S.; Lee, J.S.; Kim, Y.S.; Kim, B.S. Useful Endoscopic Ultrasonography Parameters and a Predictive Model for the Recurrence of Esophageal Varices and Bleeding after Variceal Ligation. Gut Liver 2017, 11, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Seicean, A. Endoscopic ultrasound in the diagnosis and treatment of upper digestive bleeding: A useful tool. J. Gastrointest. Liver Dis. 2013, 22, 465–469. [Google Scholar]
- Nagamine, N.; Ueno, N.; Tomiyama, T.; Aizawa, T.; Tano, S.; Wada, S.; Suzuki, T.; Amagai, K.; Ono, K.; Kumakura, Y.; et al. A Pilot Study on Modified Endoscopic Variceal Ligation Using Endoscopic Ultrasonography with Color Doppler Function. Am. J. Gastroenterol. 1998, 93, 150–155. [Google Scholar] [CrossRef] [PubMed]
- De Paulo, G.A.; Ardengh, J.C.; Nakao, F.S.; Ferrari, A.P. Treatment of esophageal varices: A randomized controlled trial comparing endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. Gastrointest. Endosc. 2006, 63, 396–402. [Google Scholar] [CrossRef]
- Sarin, S.K.; Lahoti, D.; Saxena, S.P.; Murthy, N.S.; Makwana, U.K. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology 1992, 16, 1343–1349. [Google Scholar] [CrossRef]
- Choudhuri, G.; Dhiman, R.K.; Agarwal, D.K. Endosonographic evaluation of the venous anatomy around the gas-tro-esophageal junction in patients with portal hypertension. Hepatogastroenterology 1996, 43, 1250–1255. [Google Scholar] [PubMed]
- Lee, Y.T.; Chan, F.K.; Ching, J.Y.L.; Lai, C.W.; Leung, V.K.S.; Chung, S.C.S.; Sung, J.J.Y. Diagnosis of Gastroesophageal Varices and Portal Collateral Venous Abnormalities by Endosonography in Cirrhotic Patients. Endoscopy 2002, 34, 391–398. [Google Scholar] [CrossRef]
- Rana, S.S.; Bhasin, D.K.; Singh, K. Duodenal Varix Diagnosed by Endoscopic Ultrasound. Clin. Gastroenterol. Hepatol. 2010, 8, A24. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-J. Usefulness of Endoscopic Ultrasound in Esophagogastric Varices. Clin. Endosc. 2012, 45, 324–327. [Google Scholar] [CrossRef]
- Thiruvengadam, S.S.; Sedarat, A. The Role of Endoscopic Ultrasound (EUS) in the Management of Gastric Varices. Curr. Gastroenterol. Rep. 2021, 23, 1. [Google Scholar] [CrossRef]
- Schiano, T.D.; Adrain, A.L.; Vega, K.J.; Liu, J.-B.; Black, M.; Miller, L.S. High-resolution endoluminal sonography assessment of the hematocystic spots of esophageal varices. Gastrointest. Endosc. 1999, 49, 424–427. [Google Scholar] [CrossRef]
- Irisawa, A.; Shibukawa, G.; Takagi, T.; Hikichi, T.; Obara, K.; Ohira, H.; Imamura, H. Echo-endoscopic analysis of variceal hemodynamics in patient with isolated gastric varices. Endosc. Ultrasound 2014, 3, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.S.; Teoh, A.Y.; Lau, J.Y. EUS-guided cyanoacrylate injection for treatment of endoscopically obscured bleeding gastric varices. Gastrointest. Endosc. 2015, 83, 1032–1033. [Google Scholar] [CrossRef]
- Ma, L.; Tseng, Y.; Luo, T.; Wang, J.; Lian, J.; Tan, Q.; Li, F.; Chen, S. Risk stratification for secondary prophylaxis of gastric varices due to portal hypertension. Dig. Liver Dis. 2019, 51, 1678–1684. [Google Scholar] [CrossRef]
- Lee, Y.T.; Chan, F.K.; Ng, E.K.; Leung, V.K.; Law, K.B.; Yung, M.Y.; Chung, S.; Sung, J.J.Y. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest. Endosc. 2000, 52, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.M.; Weilert, F.; Fredrick, R.T.; Kane, S.D.; Shah, J.N.; Hamerski, C.M.; Binmoeller, K.F. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: A large U.S. experience over 6 years (with video). Gastrointest. Endosc. 2016, 83, 1164–1172. [Google Scholar] [CrossRef] [Green Version]
- Romero-Castro, R.; Pellicer-Bautista, F.J.; Jimenez-Saenz, M.; Marcos-Sanchez, F.; Caunedo-Alvarez, A.; Ortiz-Moyano, C.; Gomez-Parra, M.; Herrerias-Gutierrez, J.M. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: Results in 5 cases. Gastrointest. Endosc. 2007, 66, 402–407. [Google Scholar] [CrossRef]
- Franco, M.C.; Gomes, G.F.; Nakao, F.S.; De Paulo, G.A.; Jr, A.P.F.; Jr, E.D.L. Efficacy and safety of endoscopic prophylactic treatment with undiluted cyanoacrylate for gastric varices. World J. Gastrointest. Endosc. 2014, 6, 254–259. [Google Scholar] [CrossRef]
- Romero-Castro, R.; Ellrichmann, M.; Ortiz-Moyano, C.; Subtil-Inigo, J.C.; Junquera-Florez, F.; Gornals, J.B.; Repiso-Ortega, A.; Vila-Costas, J.; Marcos-Sanchez, F.; Muñoz-Navas, M.; et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: A multicenter study (with videos). Gastrointest. Endosc. 2013, 78, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.-M.; Giacino, C.; Pioche, M.; Vanbiervliet, G.; Brardjanian, S.; Ah-Soune, P.; Vitton, V.; Grimaud, J.-C.; Barthet, M. Endoscopic ultrasound-guided vascular therapy: Is it safe and effective? Endoscopy 2012, 44, 539–542. [Google Scholar] [CrossRef]
- Bick, B.L.; Al-Haddad, M.; Liangpunsakul, S.; Ghabril, M.S.; DeWitt, J.M. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg. Endosc. 2018, 33, 1837–1845. [Google Scholar] [CrossRef] [Green Version]
- Binmoeller, K.F.; Weilert, F.; Shah, J.N.; Kim, J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest. Endosc. 2011, 74, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.C. Mechanical Devices for Arterial Occlusion and Therapeutic Vascular Occlusion Utilizing Steel Coil Technique: Clinical Applications. Am. J. Roentgenol. 2009, 192, 321–324. [Google Scholar] [CrossRef]
- Romero-Castro, R.; Pellicer-Bautista, F.; Giovannini, M.; Marcos-Sánchez, F.; Caparros-Escudero, C.; Jiménez-Sáenz, M.; Gomez-Parra, M.; Arenzana-Seisdedos, A.; Leria-Yebenes, V.; Herrerias-Gutiérrez, J.M. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy 2010, 42, E35–E36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoury, T.; Massarwa, M.; Daher, S.; Benson, A.A.; Hazou, W.; Israeli, E.; Jacob, H.; Epstein, J.; Safadi, R. Endoscopic Ultrasound-Guided Angiotherapy for Gastric Varices: A Single Center Experience. Hepatol. Commun. 2018, 3, 207–212. [Google Scholar] [CrossRef]
- Mosquera-Klinger, G.; De La Serna, C.; Bazaga, S.; García-Alonso, J.; Calero-Aguilar, H.; De Benito, M.; Hernández, R.S.-O.; Perez-Miranda, M. Obliteration of Gastric Varices Guided by Eco-Endoscopy with Coils Insertion Coated with Expandable Hidrogel Polymers. Rev. Española De Enferm. Dig. 2020, 113, 352–355. [Google Scholar] [CrossRef]
- Fujii-Lau, L.L.; Law, R.; Song, L.M.W.K.; Gostout, C.J.; Kamath, P.S.; Levy, M.J. Endoscopic ultrasound (EUS)-guided coil injection therapy of esophagogastric and ectopic varices. Surg. Endosc. 2016, 30, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Mukkada, R.J.; Antony, R.; Chooracken, M.J.; Francis, J.V.; Chettupuzha, A.P.; Mathew, P.G.; Augustine, P.; Koshy, A. Endoscopic ultrasound-guided coil or glue injection in post-cyanoacrylate gastric variceal re-bleed. Indian J. Gastroenterol. 2018, 37, 153–159. [Google Scholar] [CrossRef]
- Lôbo, M.R.D.A.; Chaves, D.M.; De Moura, D.T.H.; Ribeiro, I.B.; Ikari, E.; De Moura, E.G.H. Safety and efficacy of eus-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: A randomized controlled trial. Arq. De Gastroenterol. 2019, 56, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, S.; Pawlak, K.; Błaszczyk, Ł.; Jagielski, M.; Wiechowska-Kozłowska, A. Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. J. Clin. Med. 2019, 8, 1786. [Google Scholar] [CrossRef] [Green Version]
- Kouanda, A.; Binmoeller, K.; Hamerski, C.; Nett, A.; Bernabe, J.; Shah, J.; Bhat, Y.; Watson, R. Safety and efficacy of EUS-guided coil and glue injection for the primary prophylaxis of gastric variceal hemorrhage. Gastrointest. Endosc. 2021, 94, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.W.; Hebbar, S. EUS-guided thrombin injection for management of gastric fundal varices. Endosc. Int. Open 2018, 6, E664–E668. [Google Scholar] [CrossRef] [Green Version]
- Bazarbashi, A.N.; Wang, T.J.; Thompson, C.C.; Ryou, M. Endoscopic ultrasound-guided treatment of gastric varices with coil embolization and absorbable hemostatic gelatin sponge: A novel alternative to cyanoacrylate. Endosc. Int. Open 2020, 8, E221–E227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, P.S.; Bazarbashi, A.N.; Thompson, C.C.; Ryou, M. Successful EUS-guided treatment of gastric varices with coil embolization and injection of absorbable gelatin sponge. VideoGIE 2019, 4, 154–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.; Bhasin, D.; Chaudhary, V.; Sharma, V.; Sharma, R.; Singh, K. Clinical, endoscopic and endoscopic ultrasound features of duodenal varices: A report of 10 cases. Endosc. Ultrasound 2014, 3, 54–57. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, V.; Hijioka, S.; Hara, K.; Mizuno, N.; Imaoka, H.; Yamao, K. Endoscopic ultrasound description of liver segmentation and anatomy. Dig. Endosc. 2013, 26, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Feng, J.C.; Chang, K.J. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest. Endosc. 1999, 50, 357–361. [Google Scholar] [CrossRef]
- Tenberge, J.; Hoffman, B.J.; Hawes, R.H.; van Enckevort, C.; Giovannini, M.; Erickson, R.A.; Catalano, M.F.; Fogel, R.; Mallery, S.; Faigel, D.O.; et al. EUS-guided fine needle aspiration of the liver: Indications, yield, and safety based on an international survey of 167 cases. Gastrointest. Endosc. 2002, 55, 859–862. [Google Scholar] [CrossRef]
- DeWitt, J.M.; Leblanc, J.K.; McHenry, L.; Ciaccia, D.; Imperiale, T.; Chappo, J.; Cramer, H.; McGreevy, K.; Chriswell, M.; Sherman, S. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: A large single-center experience. Am. J. Gastroenterol. 2003, 98, 1976–1981. [Google Scholar] [CrossRef]
- Hollerbach, S.; Willert, J.; Topalidis, T.; Reiser, M.; Schmiegel, W.-H. Endoscopic Ultrasound-Guided Fine-Needle Aspiration Biopsy of Liver Lesions: Histological and Cytological Assessment. Endoscopy 2003, 35, 743–749. [Google Scholar] [CrossRef]
- McGrath, K.; Brody, D.; Luketich, J.D.; Khalid, A. Detection of Unsuspected Left Hepatic Lobe Metastases During EUS Staging of Cancer of the Esophagus and Cardia. Am. J. Gastroenterol. 2006, 101, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Crowe, D.R.; Eloubeidi, M.A.; Chhieng, D.C.; Jhala, N.C.; Jhala, D.; Eltoum, I.A. Fine-needle aspiration biopsy of hepatic lesions: Computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer 2006, 108, 180–185. [Google Scholar] [CrossRef]
- Singh, P.; Erickson, R.A.; Mukhopadhyay, P.; Gopal, S.; Kiss, A.; Khan, A.; Westblom, T.U. EUS for detection of the hepatocellular carcinoma: Results of a prospective study. Gastrointest. Endosc. 2007, 66, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mukhopadhyay, P.; Bhatt, B.; Patel, T.; Kiss, A.; Gupta, R.; Bhat, S.; Erickson, R.A. Endoscopic Ultrasound Versus CT Scan for Detection of the Metastases to the Liver: Results of a prospective comparative study. J. Clin. Gastroenterol. 2009, 43, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Prachayakul, V.; Aswakul, P.; Kachintorn, U. EUS guided fine needle aspiration cytology of liver nodules suspicious for ma-lignancy: Yields, complications and impact on management. J. Med. Assoc Thai 2012, 95, S56–S60. [Google Scholar] [PubMed]
- Lee, Y.N.; Moon, J.H.; Kim, H.K.; Choi, H.J.; Choi, M.H.; Kim, D.C.; Lee, T.H.; Cha, S.-W.; Kim, S.G.; Kim, Y.S. Usefulness of endoscopic ultrasound-guided sampling using core biopsy needle as a percutaneous biopsy rescue for diagnosis of solid liver mass: Combined histological-cytological analysis. J. Gastroenterol. Hepatol. 2015, 30, 1161–1166. [Google Scholar] [CrossRef]
- Seo, D.-W.; Oh, D.; Hong, S.-M.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Endoscopic ultrasound-guided fine-needle aspiration can target right liver mass. Endosc. Ultrasound 2017, 6, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Minaga, K.; Kitano, M.; Nakai, A.; Omoto, S.; Kamata, K.; Yamao, K.; Takenaka, M.; Tsurusaki, M.; Chikugo, T.; Matsumoto, I.; et al. Improved detection of liver metastasis using Kupffer-phase imaging in contrast-enhanced harmonic EUS in patients with pancreatic cancer (with video). Gastrointest. Endosc. 2021, 93, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Moon, J.H.; Kim, H.K.; Lee, Y.N.; Lee, T.H.; Cha, S.; Cho, Y.D.; Park, S. KRASmutation analysis by next-generation sequencing in endoscopic ultrasound-guided sampling for solid liver masses. J. Gastroenterol. Hepatol. 2017, 32, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Ichim, V.A.; Chira, R.I.; Mircea, P.A. Diagnostic yield of endoscopic ultrasound-guided biopsy of focal liver lesions. Med. Pharm. Rep. 2019, 92, 15–20. [Google Scholar] [CrossRef]
- Chon, H.K.; Yang, H.C.; Choi, K.H.; Kim, T.H. Endoscopic Ultrasound-Guided Liver Biopsy Using a Core Needle for Hepatic Solid Mass. Clin. Endosc. 2019, 52, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Akay, E.; Atasoy, D.; Altınkaya, E.; Koç, A.; Ertan, T.; Karaman, H.; Caglar, E. Endoscopic Ultrasound-Guided Fine Needle Aspiration Using a 22-G Needle for Hepatic Lesions: Single-Center Experience. Clin. Endosc. 2021, 54, 404–412. [Google Scholar] [CrossRef]
- Sbeit, W.; Kadah, A.; Mahamid, M.; Pellicano, R.; Mari, A.; Khoury, T. A State-of-the-Art Review on the Evolving Utility of Endoscopic Ultrasound in Liver Diseases Diagnosis. Diagnostics 2020, 10, 512. [Google Scholar] [CrossRef]
- Midia, M.; Devang, O.; Shuster, A.; Midia, R.; Muir, J.; Odedra, D. Predictors of bleeding complications following percutaneous image-guided liver biopsy: A scoping review. Diagn. Interv. Radiol. 2019, 25, 71–80. [Google Scholar] [CrossRef]
- Karsenti, D.; Palazzo, L.; Perrot, B.; Zago, J.; Lemaistre, A.-I.; Cros, J.; Napoléon, B. 22G Acquire vs. 20G Procore needle for endoscopic ultrasound-guided biopsy of pancreatic masses: A randomized study comparing histologic sample quantity and diagnostic accuracy. Endoscopy 2020, 52, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Muniraj, T.; Aslanian, H.R. Endoscopic Ultrasound for the Diagnosis and Staging of Liver Tumors. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 339–350. [Google Scholar] [CrossRef]
- Fusaroli, P.; Lisotti, A.; Serrani, M.; Caletti, G. EUS liver assessment using contrast agents and elastography. Endosc. Ultrasound 2018, 7, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, P.; Napoleon, B.; Gincul, R.; Lefort, C.; Palazzo, L.; Palazzo, M.; Kitano, M.; Minaga, K.; Caletti, G.; Lisotti, A. The clinical impact of ultrasound contrast agents in EUS: A systematic review according to the levels of evidence. Gastrointest. Endosc. 2016, 84, 587–596.e10. [Google Scholar] [CrossRef]
- Kammerer, S.; Meister, T.; Wolters, H.; Lessing, M.; Hüsing, A.; Domagk, D.; Floer, M.; Wilms, C.; Schmidt, H.; Senninger, N.; et al. Preoperative prediction of curative surgery of perihilar cholangiocarcinoma by combination of endoscopic ultrasound and computed tomography. United Eur. Gastroenterol. J. 2018, 6, 263–271. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Beyna, T.; Gerges, C. Clinical Management of Bile Duct Diseases: Role of Endoscopic Ultrasound in a Personalized Approach. J. Pers. Med. 2020, 11, 1. [Google Scholar] [CrossRef]
- Girotra, M.; Soota, K.; Dhaliwal, A.S.; Abraham, R.R.; Garcia-Saenz-De-Sicilia, M.; Tharian, B. Utility of endoscopic ultrasound and endoscopy in diagnosis and management of hepatocellular carcinoma and its complications: What does endoscopic ultrasonography offer above and beyond conventional cross-sectional imaging? World J. Gastrointest. Endosc. 2018, 10, 56–68. [Google Scholar] [CrossRef]
- Gonzalo-Marín, J.; Vila, J.J.; Perez-Miranda, M. Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. World J. Gastrointest. Oncol. 2014, 6, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Kujundzic, M.; Stoos-Veic, T.; Kaic, G.; Vukelic-Markovic, M. Role of Repeated Endoscopic Ultrasound-Guided Fine Needle Aspiration in Small Solid Pancreatic Masses with Previous Indeterminate and Negative Cytological Findings. Dig. Dis. 2008, 26, 377–382. [Google Scholar] [CrossRef]
- Smith, I.; Monkemuller, K.; Wilcox, C.M. Incidentally Identified Common Bile Duct Dilatation: A Systematic Review of Eval-uation, Causes, and Outcome. J. Clin. Gastroenterol. 2015, 49, 810–815. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mohamadnejad, M.; Islami, F.; Keshtkar, A.; Biglari, M.; Malekzadeh, R.; Eloubeidi, M.A. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: A systematic review and meta-analysis. Gastrointest. Endosc. 2016, 83, 290–298.e1. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78. [Google Scholar] [CrossRef]
- Phan, J.; Ge, P.S.; Kardashian, A.; Kim, S.; Sedarat, A.; Watson, R.; Muthusamy, V.R. The role of endoscopic ultrasound in evaluating patients with bile duct dilation of unclear etiology. J. Dig. Dis. 2021, 22, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Seo, D.-W.; Paik, W.H.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Ethanol lavage of huge hepatic cysts by using EUS guidance and a percutaneous approach. Gastrointest. Endosc. 2014, 80, 1014–1021. [Google Scholar] [CrossRef]
- Keohane, J.; DiMaio, C.J.; Schattner, M.A.; Gerdes, H. EUS-guided transgastric drainage of caudate lobe liver abscesses. J. Interv. Gastroenterol. 2011, 1, 139–141. [Google Scholar]
- Noh, S.H.; Park, D.H.; Kim, Y.R.; Chun, Y.; Lee, H.C.; Lee, S.O.; Seo, D.W.; Lee, S.K.; Kim, M.-H. EUS-guided drainage of hepatic abscesses not accessible to percutaneous drainage (with videos). Gastrointest. Endosc. 2010, 71, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Seewald, S.; Imazu, H.; Omar, S.; Groth, S.; Seitz, U.; Brand, B.; Zhong, Y.; Sikka, S.; Thonke, F.; Soehendra, N. EUS-guided drainage of hepatic abscess. Gastrointest. Endosc. 2005, 61, 495–498. [Google Scholar] [CrossRef]
- Kumta, N.A.; Torres-Ruiz, F.; Reinoso, P.J.; Kahaleh, M. Endoscopic management of hepatic abscess after EUS-guided hepaticogastrostomy. Gastrointest. Endosc. 2016, 84, 1054–1055. [Google Scholar] [CrossRef]
- Itoi, T.; Ang, T.L.; Seewald, S.; Tsuji, S.; Kurihara, T.; Tanaka, R.; Itokawa, F. Endoscopic ultrasonography-guided drainage for tuberculous liver abscess drainage. Dig. Endosc. 2011, 23, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Masuda, S.; Uojima, H.; Ichita, C.; Tokoro, S.; Sasaki, A.; Egashira, H.; Kimbara, T.; Kako, M. Endoscopic ultrasound-guided drainage of an amoebic liver abscess extending into the hepatic subcapsular space. Clin. J. Gastroenterol. 2015, 8, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Takagi, W.; Onda, S.; Masuda, D.; Kitano, M.; Imoto, A.; Higuchi, K. Endoscopic ultrasound-guided drainage of a right liver abscess with a self-expandable metallic stent. Endoscopy 2015, 47, E397–E398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaide, N.; Vargas-Garcia, A.L.; De La Serna-Higuera, C.; Del Val, L.S.; Ruiz-Zorrilla, R.; Perez-Miranda, M. EUS-guided drainage of liver abscess by using a lumen-apposing metal stent (with video). Gastrointest. Endosc. 2013, 78, 941–942. [Google Scholar] [CrossRef] [PubMed]
- Tonozuka, R.; Itoi, T.; Tsuchiya, T.; Sofuni, A.; Ishii, K.; Ikeuchi, N.; Umeda, J.; Tanaka, R.; Mukai, S.; Gotoda, T.; et al. EUS-guided drainage of hepatic abscess and infected biloma using short and long metal stents (with videos). Gastrointest. Endosc. 2015, 81, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Masuda, D.; Saori, O.; Wataru, T.; Sano, T.; Okuda, A.; Miyano, A.; Kitano, M.; Abdel-Aal, U.M.; Takeuchi, T.; et al. Clinical Outcome of Endoscopic Ultrasound-Guided Liver Abscess Drainage Using Self-Expandable Covered Metallic Stent (with Video). Dig. Dis. Sci. 2015, 61, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Medrado, B.F.; Carneiro, F.O.A.A.; Vilaça, T.G.; Gouveia, T.S.; Frazão, M.S.V.; de Moura, E.; Sakai, P.; Otoch, J.P.; Artifon, E.L.A. Endoscopic ultrasound-guided drainage of giant liver abscess associated with transgastric migration of a self-expandable metallic stent. Endoscopy 2013, 45, E331–E332. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, H.; Kawakubo, K.; Kuwatani, M.; Kubota, Y.; Abe, Y.; Kawahata, S.; Kubo, K.; Sakamoto, N. Endoscopic ultrasonography-guided liver abscess drainage using a dedicated, wide, fully covered self-expandable metallic stent with flared-ends. Endoscopy 2014, 46, E982–E983. [Google Scholar] [CrossRef] [Green Version]
- Carbajo, A.Y.; Vegas, F.J.B.; García-Alonso, F.J.; Cimavilla, M.; Yuste, R.T.; Gil Simón, P.; Serna-Higuera, C.; Pérez, G.C.F.; Pérez-Miranda, M.; De La Serna-Higuera, C.; et al. Retrospective cohort study comparing endoscopic ultrasound-guided and percutaneous drainage of upper abdominal abscesses. Dig. Endosc. 2019, 31, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.K.; Asokkumar, R. Endoscopic ultrasound-guided drainage of difficult-to-access liver abscesses. SAGE Open Med. 2020, 8, 205031212092127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.-A.; Deng, Z.; Tian, G.; Zhao, Q.-Y.; Wang, W.-L. Efficacy and safety of endoscopic ultrasonography-guided interventional treatment for refractory malignant left-sided liver tumors: A case series of 26 patients. Sci. Rep. 2016, 6, 36098. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, S.; Hirata, N.; Mikata, R.; Kobayashi, M.; Shiratori, T.; Ogasawara, S.; Ooka, Y.; Tsuyuguchi, T.; Yamaguchi, T.; Yokosuka, O. Clinical outcomes of endoscopic ultrasound-guided ethanol injection for hepatocellular carcinoma in the caudate lobe. Endosc. Int. Open 2016, 4, E1111–E1115. [Google Scholar] [CrossRef] [Green Version]
- Nakaji, S.; Hirata, N.; Kobayashi, M.; Shiratori, T.; Sanagawa, M. Endoscopic ultrasonography-guided ethanol injection as a treatment for ruptured hepatocellular carcinoma in the left hepatic lobe. Endoscopy 2015, 47, E558–E560. [Google Scholar] [CrossRef] [Green Version]
- Lisotti, A.; Piscaglia, F.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided ethanol injection for a small hepatocellular carcinoma. Endoscopy 2019, 51, E317–E318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaji, S.; Hirata, N.; Iwaki, K.; Shiratori, T.; Kobayashi, M.; Inase, M. Endoscopic ultrasound (EUS)-guided ethanol injection for hepatocellular carcinoma difficult to treat with percutaneous local treatment. Endoscopy 2011, 44, E380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, R.L.; Perez-Miranda, M.; Giovannini, M. EUS-guided treatment of a solid hepatic metastasis. Gastrointest. Endosc. 2002, 55, 266–270. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Tuo, X.-P.; Jin, Z.-D.; Liu, Y.; Guo, Y.; Luo, L. Endoscopic ultrasound (EUS)-guided ethanol injection in hepatic metastatic carcinoma: A case report. Endoscopy 2010, 42, E256–E257. [Google Scholar] [CrossRef] [Green Version]
- Faigel, D.O.; Singh, V.P.; Patel, K.; El Chami, A.; Raymond, C.C.; Landreth, T.L.; Marler, R.J.; Lake, D.F.; Kachaamy, T. Safety of Endoscopic-Ultrasound-Guided Portal Injection Chemotherapy using Drug-Eluting Microbeads in a Porcine Model. J. Cancer Res. Updat. 2018, 7, 102–108. [Google Scholar] [CrossRef]
- Faigel, U.O.; Lake, D.F.; Landreth, T.L.; Kelman, C.C.; Marler, R.J.; Information, P.E.K.F.C. EUS-guided portal injection chemotherapy for treatment of hepatic metastases: Feasibility in the acute porcine model. Gastrointest. Endosc. 2015, 83, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Hines-Peralta, A.; Hollander, C.Y.; Solazzo, S.; Horkan, C.; Liu, Z.-J.; Goldberg, S.N. Hybrid Radiofrequency and Cryoablation Device: Preliminary Results in an Animal Model. J. Vasc. Interv. Radiol. 2004, 15, 1111–1120. [Google Scholar] [CrossRef]
- Di Matteo, F.; Grasso, R.F.; Pacella, C.M.; Martino, M.; Pandolfi, M.; Rea, R.; Luppi, G.; Silvestri, S.; Zardi, E.; Costamagna, G. EUS-guided Nd:YAG laser ablation of a hepatocellular carcinoma in the caudate lobe. Gastrointest. Endosc. 2011, 73, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Pioche, M.; Lafon, C.; Constanciel, E.; Vignot, A.; Birer, A.; Gincul, R.; Lépilliez, V.; Prat, F.; Roman, S.; Chapelon, J.-Y.; et al. High-intensity focused ultrasound liver destruction through the gastric wall under endoscopic ultrasound control: First experience in living pigs. Endoscopy 2012, 44, E376–E377. [Google Scholar] [CrossRef] [Green Version]
- Armellini, E.; Leutner, M.; Stradella, D.; Ballarè, M.; Occhipinti, P. EUS-guided radiofrequency ablation: An option for the extrapancreatic region. Endosc. Ultrasound 2018, 7, 282–283. [Google Scholar] [CrossRef]
- Jiang, T.; Tian, G.; Bao, H.; Chen, F.; Deng, Z.; Li, J.; Chai, W. EUS dating with laser ablation against the caudate lobe or left liver tumors: A win-win proposition? Cancer Biol. Ther. 2018, 19, 145–152. [Google Scholar] [CrossRef]
- Choi, J.-H.; Seo, D.-W.; Park, D.H.; Lee, S.K.; Kim, M.-H. Fiducial Placement for Stereotactic Body Radiation Therapy under Only Endoscopic Ultrasonography Guidance in Pancreatic and Hepatic Malignancy: Practical Feasibility and Safety. Gut Liver 2014, 8, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Dhadham, G.C.; Hoffe, S.; Harris, C.L.; Klapman, J.B. Endoscopic ultrasound-guided fiducial marker placement for image-guided radiation therapy without fluoroscopy: Safety and technical feasibility. Endosc. Int. Open 2016, 04, E378–E382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.-H.; Nishioka, N.S.; Mino, M.; Lauwers, G.Y.; Puricelli, W.P.; Collier, K.N.; Brugge, W.R. EUS-guided photodynamic therapy of the pancreas: A pilot study. Gastrointest. Endosc. 2004, 59, 95–99. [Google Scholar] [CrossRef]
- Lutz, H.H.; Wasmuth, H.E.; Streetz, K.; Tacke, F.; Koch, A.; Luedde, T.; Trautwein, C.; Tischendorf, J.J.W. Endoscopic ultrasound as an early diagnostic tool for primary sclerosing cholangitis: A prospective pilot study. Endoscopy 2012, 44, 934–939. [Google Scholar] [CrossRef]
- Tischendorf, J.J.W.; Hecker, H.; Krüger, M.; Manns, M.P.; Meier, P.N. Characterization, Outcome, and Prognosis in 273 Patients with Primary Sclerosing Cholangitis: A Single Center Study. Am. J. Gastroenterol. 2007, 102, 107–114. [Google Scholar] [CrossRef]
- Mesenas, S.; Vu, C.; Doig, L.; Meenan, J. Duodenal EUS to identify thickening of the extrahepatic biliary tree wall in primary sclerosing cholangitis. Gastrointest. Endosc. 2006, 63, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Rustemovic, N.; Cukovic-Cavka, S.; Opacic, M.; Petrovecki, M.; Hrstic, I.; Radic, D.; Ostojic, R.; Pulanic, R.; Vucelic, B. Endoscopic ultrasound elastography as a method for screening the patients with suspected primary sclerosing cholangitis. Eur. J. Gastroenterol. Hepatol. 2009, 22, 748–753. [Google Scholar] [CrossRef] [PubMed]
- De Moura, D.H.; De Moura, E.H.; Bernardo, W.; De Moura, E.H.; Baracat, F.; Kondo, A.; Matuguma, S.; Artifon, E.A. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc. Ultrasound 2018, 7, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, F.C.; Rajan, E.; Levy, M.J.; Clain, J.E.; Topazian, M.D.; Harewood, G.C.; Papachristou, G.I.; Takahashi, N.; Rosen, C.B.; Gores, G.J. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest. Endosc. 2008, 67, 438–443. [Google Scholar] [CrossRef] [PubMed]

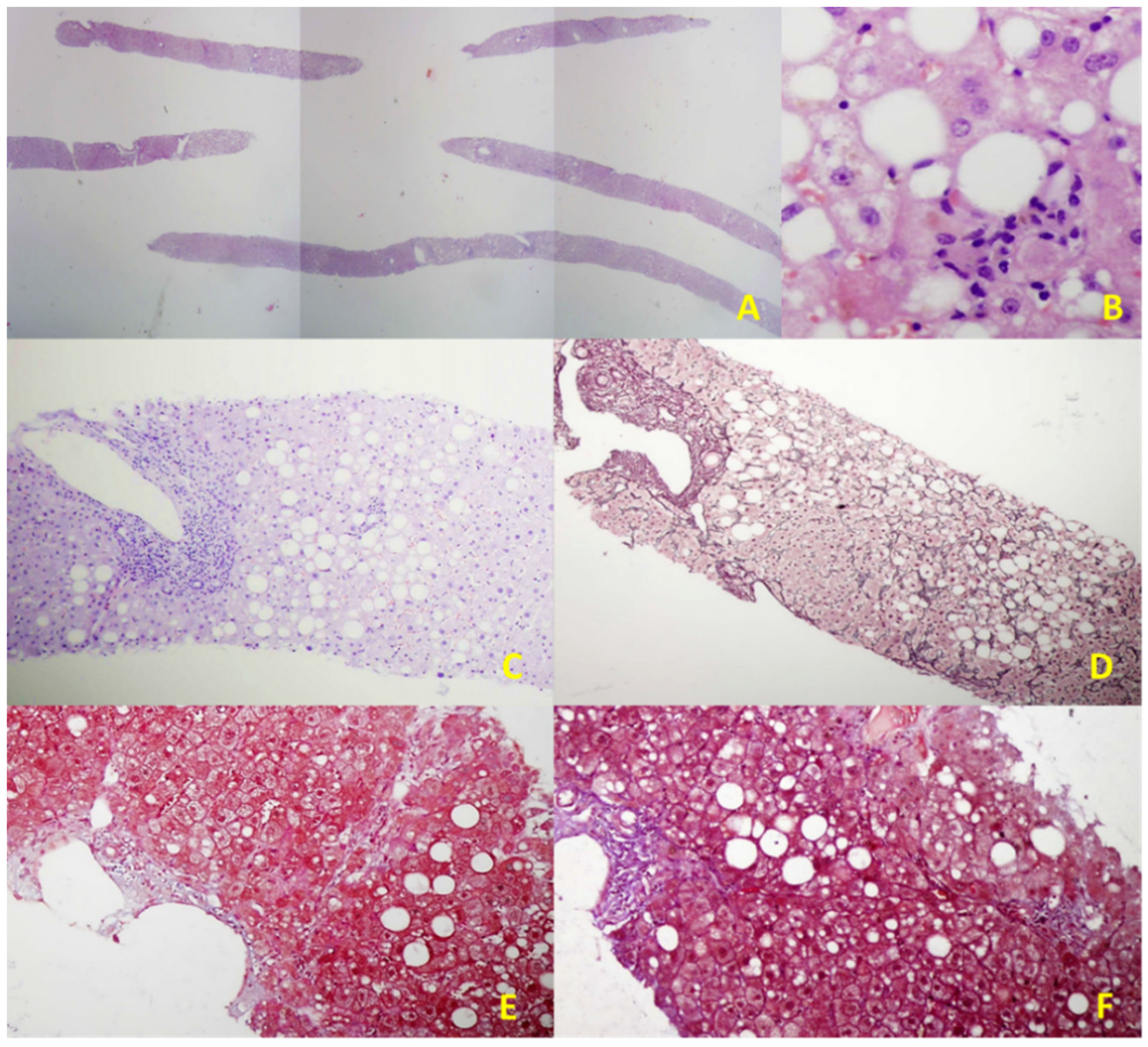
| Reference/Study Design | Number of Patients | Type of Needle | Number of Passes (Range) | Technical Success (%) | Diagnostic Yield (%) | Specimen Length (mm; Median, Range) | Complete Portal Tracts (Median, Range) | Fragmentation of the Specimen (%) | Complications, N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mathew et al., 2007 [22] case report | 2 | Quickcore/19G | 2 | 100 | 100 | range 6–11 | 0 | ||
| Gleeson et al., 2008 [23] retrospective case series | 9 | Quickcore/19G | 2 (1–3) | 100 | 100 | 16.9 (8–28) | 7 (5–8) | 0 | |
| DeWitt et al., 2009 [24] prospective case series | 21 | Quickcore/19G | 3 (1–4) | 100 | 90 | 9 (1–23) | 2 (0–10) | 29 | 0 |
| Stavropoulos et al., 2012 [26] prospective case series | 22 | 19G FNA (EchoTip) | 2 (1–3) | 91 | 91 | 36.9 (2–184.6) | 9 (1–73) | 4.5 | 0 |
| Gor et al., 2014 [30] prospective case series | 10 | 19G FNA (Expect) | 3 | 100 | 100 | 14.4 (6–22) | 9.2 (6–15) | 0 | |
| Diehl et al., 2015 [25] prospective multicenter study | 110 | 19G FNA (Expect) | 1–2 | 100 | 98 | 38 (0–203) | 14 (0–68) | 1 (0.9) pericapsular hematoma | |
| DeWitt et al., 2015 prospective case series | 44 | 19G FNB (Procore) | 1–3 | 95 | 88 | 15 (3–60) | mean 10.4 ± 4.7 | 6 (14.6) | |
| 41 | 19G FNB (Quick-Core) | 1–3 | 78 | 62 | 3 (0–14) | mean 1.3 ± 1.9 | 8 (21.6) | ||
| Pineda et al., 2016 [31] retrospective study | 110 | 19G FNA (Expect) | 1–4 | 100 | 100 | 38 (24–81) | 14 (9–27) | none reported | |
| Sey et al., 2016 [32] cross-sectional study | 30 | 19G FNB (Procore) | 2 (1–3) | 100 | 97 | 20 (5–60) | 5 (0–24) | 0 | |
| 45 | 19G FNB (Quick-Core) | 3 (1–7) | 98 | 73 | 9 (0–25) | 2 (0–15) | 2 (4.4) | ||
| Saab et al., 2017 [33] retrospective study | 47 | 19GFNB (Sharkcore) | modified 1 pass | 100 | 100 | 65 (46–80) | 18 (14–24) | 2 (4.2) hematoma | |
| Shah et al., 2017 [34] retrospective study | 24 | 19GFNB (Sharkcore) | 2 (1–3) | 100 | 96 | 65.6 (17–167.4) | 32.5 (5–85) | 2 (8.3) pain, subcapsular bleeding | |
| Mok et al., 2018 [35] prospective crossover study | 40 dry control | 19G FNA (Expect Flexible) | 1 | 100 | 80 | 23.9 (12.3–54.2) | 4 (2–10) | 1 (2.5) bleeding | |
| 40 dry heparin | 1 | 92.5 | 29.7 (18.5–56.3) | 4 (2–6) | |||||
| 40 wet heparin | 1 | 97.5 | 49.2 (32.8–68.4) | 7 (5–12) | |||||
| Nieto et al., 2018 [36] retrospective study | 165 | 19GFNB (Sharkcore) | 1 | 100 | 100 | 60 (43–80) | 18 (13–24) | 3 (1.8) pain, hematoma | |
| Ching-Compagnioni et al., 2019 [37] prospective randomized trial | 20 | 19G FNA (ExpectFlexible) | 2 | 100 | 100 | mean 114 | 16.5 (6–38) | 8 (40) pain | |
| 20 | 19G FNB (Acquire) | mean 153 | 38 (0–81) | 7 (35) pain | |||||
| Hasan et al., 2019 [38] prospective study | 40 | 22G FNB (Acquire) | 3 | 100 | 100 | 55 (44.5–68) | 42 (28.5–53) | 6 (15) pain | |
| Bazerbachi et al., 2019 [39] prospective study | 21 | 22G FNB (SharkCore) | 2 | 100 | 100 | 24 (20–27.5) | 26 (7–62) | 3 (14.2) pain | |
| Mok et al., 2019 [40] randomized crossover study | 40 | 19G FNA (ExpectFlexible) | 2 | 100 | 88 | mean 61 | mean 7.4 | 80 small fragments (% of total) | 1 (1.2) pain |
| 40 | 22G FNB (SharkCore) | 2 | 68 | mean 48.1 | mean 6.1 | 90 | |||
| Khurana et al., 2019 [41] retrospective study | 38 | 19G FNA (Expect) | 2 | 100 | 100 | range 12–133 | range 5–68 for left; 6–29 for right lobe | none reported | |
| Shuja et al., 2019 [42] retrospective study | 69 | 19G FNA (ExpectFlexible) | median 3 | 100 | 100 | mean 45.8 | mean 10.8 | 72 | 0 |
| Aggarwal et al.,2020 [43] Prospective study | 108 | 19G FNB (SharkCore) | 2 | 100 | 79.4 | mean 13.86 | mean 7.07 | 52.4 | 1 (0.9) |
| 19G FNB (Acquire) | 97.2 | mean 15.81 | mean 9.59 | 24.8 | |||||
| Nieto et al., 2020 [44] retrospective study | 210 | 19G FNB (Acquire) | 2 | 100 | 100 | mean 65 | mean 24.0 | 4 (2%) pain, 2 (1%) hematoma, bile leak | |
| 210 | 19G FNB (SharkCore) | mean 60 | mean 19.5 | 5 (17%) pain, 1 (0.5%) hematoma | |||||
| Hashimoto et al., 2020 [45] Prospective crossover study | 22 | 19G FNB (Acquire) | 1 | 100 | 100 | 19.9 (3–73) | 14.4 (2–33) | 2 (9.1) pain | |
| 19G FNB (SharkCore) | 100 | 95.4 | 13.7 (3–66) | 9.5 (0–35) | |||||
| Ali et al., 2020 [46] Retrospective study | 30 | 19G or 22G FNB (SharkCore) | 2 | 100 | 100 | 25 (21–33) | 5 (5–8) | 40 | 1 (3–3) pain |
| Patel et al., 2021 [47] | 30 | 22G FNB (Acquire) | Not standardized | 100 | 66.7 | mean 38 | mean 6.9 | not reported | |
| 50 | 19G FNB QuickCore) | 46 | mean 47 | mean 3.0 | |||||
| 28 | 19G FNB (ProCore) | 82.1 | mean 39 | mean 7.3 | |||||
| 27 | 19G FNA (Expect) | 81.5 | mean 84 | mean 16.9 | |||||
| Bang et al., 2021 [48] | 21 | 19G FNB (Acquire) | 2 (both lobes) | 100 | 100 (91.5 from single pass) | 16.5 (9.5–32.5) | ≥10 cpt 81% | 0 |
| Author (Year) | Patient Number | Study Type | Method (CYA/Coil) | Type of Treatment (Primary Prophylaxis (PP)/Acute GV Hemorrhage (AH)/Secondary Prophylaxis (SP)) | GV Obliteration Rate | Re-Bleeding Rate | Severe Complications Rate |
|---|---|---|---|---|---|---|---|
| Romero-Castro et al. (2007) [86] | 5 | Prospective | CYA | PP | 5 (100%) | 0 (0%) | 0 (0%) |
| Romero-Castro et al. (2010) [93] | 4 | Prospective | coil | PP | 3 (75%) | 0 (0%) | 0 (0%) |
| Binmoeller et al. (2011) [91] | 30 | Retrospective | CYA/coil | AH/SP | 23/24 (96%) | 4 (16.6%) (not attributed to GV) | 0 (0%) |
| Gonzalez et al. (2012) [89] | 3 | Retrospective | CYA | AH | 3 (100%) | 0 (0%) | 0 (0%) |
| Romero-Castro et al. (2013) [88] | 19 | Retrospective | CYA | AH | 18 (95%) | 0 (0%) | 0 (0%) |
| Romero-Castro et al. (2013) [88] | 11 | Retrospective | coil | PP | 10 (91%) | 0 (0%) | 0 (0%) |
| Franco et al. (2014) [87] | 20 | Prospective | CYA | PP | 20 (100%) | 1 (5%) | 0 (0%) |
| Fujii-Lau et al. (2016) [96] | 2 | Retrospective | coil | SP | 2 (100%) | 0 (0%) | N/A |
| Fujii-Lau et al. (2016) [96] | 3 | Retrospective | CYA/coil | SP | 3 (100%) | 0 (0%) | N/A |
| Bhat et al. (2016) [85] | 152 | Retrospective | CYA/coil | PP/AH | 93/100 (93%) | 20/125 (16%) | 1/125 (0.8%) (PE) |
| Mukkada et al. (2018) [97] | 30 | Retrospective | CYA/coil | AH/SP | 8/20 (40%) | 6 (20%) | N/A |
| Lobo et al. (2019) [98] | 16 | Prospective | CYA/coil | 15/15 (100%) | 0 (0%) | 4 (25%) (PE) | |
| Koziel et al. (2019) [99] | 16 | Prospective | CYA/coil | PP/SP | 15 (94%) | 0 (0%) | 0 (0%) |
| Bick et al. (2019) [90] | 64 | Prospective | CYA | AH | 49 (77%) | 5 (8%) | 3 (5%) (PE, splenic infarct) |
| Khoury et al. (2019) [94] | 10 | Prospective | coil | PP/AH | 7 (70%) | 0 (0%) | 1 (10%) (major bleeding) |
| Mosquera-Klinger et al. (2021) [95] | 4 | Retrospective | (hydro)coil | AH | 4 (100%) | 0 (0%) | 0 (0%) |
| Kouanda et al. (2021) [100] | 80 | Prospective | CYA/coil | PP | 77 (97%) | 2 (2.5%) | 4 (5%) |
| Author (Year) | Patient Number | Study Type | Tissue Acqusition Type | Needle Size | Needle Passes (Median) | Diagnostic Yield | Complications (%) |
|---|---|---|---|---|---|---|---|
| Nguyen et al. (1999) [106] | 14 | Prospective | FNA | 22G | 2 | 100 | 0 |
| tenBerge et al. (2002) [107] | 167 | Retrospective | FNA | 22G | - | 95.8 | 6 (3.6) |
| Dewitt et al. (2003) [108] | 77 | Retrospective | FNA | 22G | 3.4 (mean) | 100 | 0 |
| Hollerbach et al. (2003) [109] | 33 | Prospective | FNA | 22G | 1.4 (mean) | 94 | 2 (6.1) |
| McGrath et al. (2006) [110] | 7 | Prospective | FNA | 22G | 2 | 100 | 0 |
| Crowe et al. (2006.) [111] | 16 | Retrospective | FNA | 22G | 3 | 75 | 0 |
| Singh et al. (2007) [112] | 9 | Prospective | FNA | 22G | 2 | 88.9 | 0 |
| Singh et al. (2009) [113] | 26 | Prospective | FNA | 22G | 2.1 (mean) | 98 | 0 |
| Prachayakul et al. (2012) [114] | 14 | Retrospective | FNA | 22G | - | 100 | 0 |
| Lee et al. (2015) [115] | 21 | Prospective | FNB | FNB/20G or 22G or 25G | 2 | 90.5 | 0 |
| Oh et al. (2017.) [116] | 47 | Prospective | FNA | 22G or 25G | 3 | 90.5 | 0 |
| Minaga et al. (2017) [117] | 338 | Prospective | FNA | 22G or 25G | - | 95.8 * | 0 |
| Choi et al. (2017) [118] | 28 | Prospective | FNA | 22G or 25G | 2 | 89.3 | 0 |
| Ichim et al. (2019) [119] | 48 | Prospective | FNA | 22G | 2 | 98 | 0 |
| Chon et al. (2019) [120] | 58 | Retrospective | FNB | FNB ProCore/20G or 22G or 25G | 2 | 89.7 | 1 (1.7) |
| Akay et al. (2021) [121] | 25 | Retrospective | FNA | 22G | 1 | 95.5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavic, T.; Mikolasevic, I.; Kralj, D.; Blazevic, N.; Skrtic, A.; Budimir, I.; Lerotic, I.; Hrabar, D. Role of Endoscopic Ultrasound in Liver Disease: Where Do We Stand? Diagnostics 2021, 11, 2021. https://doi.org/10.3390/diagnostics11112021
Pavic T, Mikolasevic I, Kralj D, Blazevic N, Skrtic A, Budimir I, Lerotic I, Hrabar D. Role of Endoscopic Ultrasound in Liver Disease: Where Do We Stand? Diagnostics. 2021; 11(11):2021. https://doi.org/10.3390/diagnostics11112021
Chicago/Turabian StylePavic, Tajana, Ivana Mikolasevic, Dominik Kralj, Nina Blazevic, Anita Skrtic, Ivan Budimir, Ivan Lerotic, and Davor Hrabar. 2021. "Role of Endoscopic Ultrasound in Liver Disease: Where Do We Stand?" Diagnostics 11, no. 11: 2021. https://doi.org/10.3390/diagnostics11112021






