Beyond the Patient’s Report: Self-Reported, Subjective, Objective and Estimated Walking Disability in Patients with Peripheral Artery Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements Collected
2.3. Statistical Analysis
3. Results
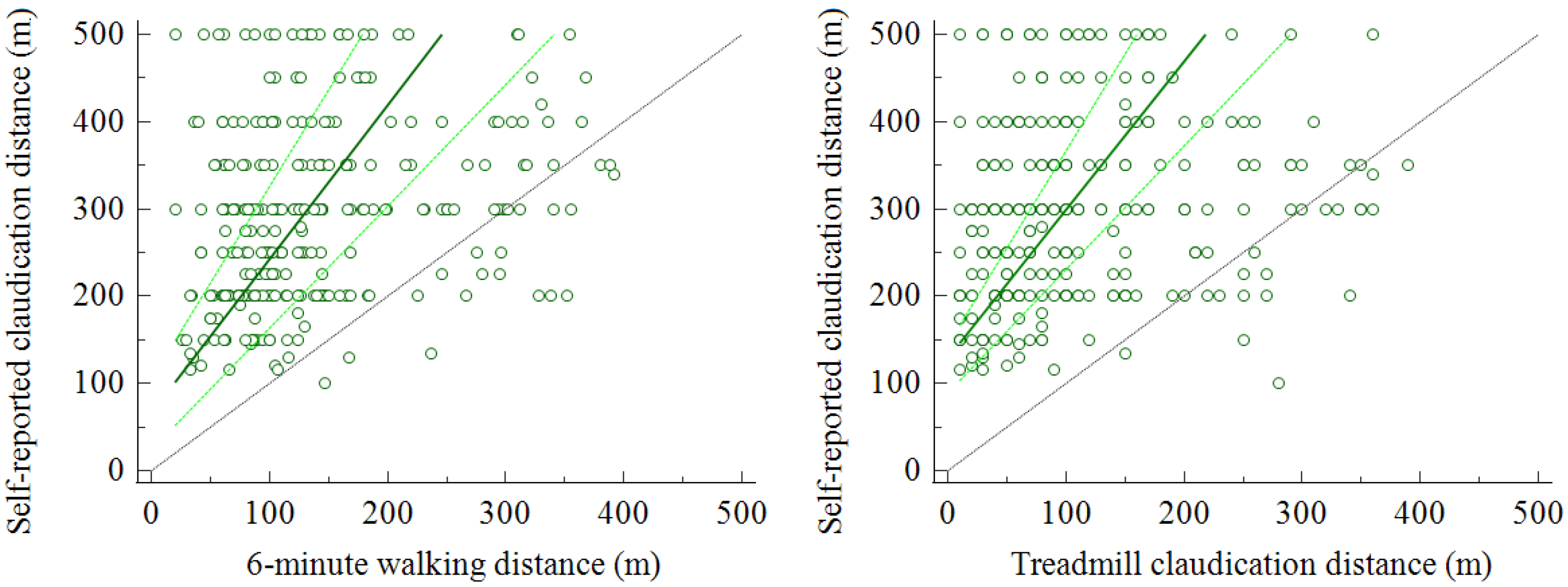
3.1. Self-Reported and Measured Walked Distances
3.2. Comparison between Self-Reported and Measured Walked Distance
3.3. Relationship with Objective Measurements
3.4. Factors Related to Walked Distance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, e71–e126. [Google Scholar] [CrossRef]
- Fowkes, F.G.R.; Aboyans, V.; Fowkes, F.J.I.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral Artery Disease: Epidemiology and Global Perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef]
- Watson, C.J.; Phillips, D.; Hands, L.; Collin, J. Claudication Distance Is Poorly Estimated and Inappropriately Measured. Br. J. Surg. 1997, 84, 1107–1109. [Google Scholar]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. 1), S5–S67. [Google Scholar] [CrossRef] [Green Version]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal. Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [Green Version]
- Frans, F.A.; Zagers, M.B.; Jens, S.; Bipat, S.; Reekers, J.A.; Koelemay, M.J.W. The Relationship of Walking Distances Estimated by the Patient, on the Corridor and on a Treadmill, and the Walking Impairment Questionnaire in Intermittent Claudication. J. Vasc. Surg. 2013, 57, 720–727.e1. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.J.E.; Collin, J. Estimates of Distance by Claudicants and Vascular Surgeons Are Inherently Unreliable. Eur. J. Vasc. Endovasc. Surg. 1998, 16, 429–430. [Google Scholar] [CrossRef] [Green Version]
- Manfredini, F.; Lamberti, N.; Malagoni, A.M.; Zambon, C.; Basaglia, N.; Mascoli, F.; Manfredini, R.; Zamboni, P. Reliability of the Vascular Claudication Reporting in Diabetic Patients with Peripheral Arterial Disease: A Study with near-Infrared Spectroscopy. Angiology 2015, 66, 365–374. [Google Scholar] [CrossRef]
- Tew, G.; Copeland, R.; Le Faucheur, A.; Gernigon, M.; Nawaz, S.; Abraham, P. Feasibility and Validity of Self-Reported Walking Capacity in Patients with Intermittent Claudication. J. Vasc. Surg. 2013, 57, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Mahe, G.; Ouedraogo, N.; Marchand, J.; Vielle, B.; Picquet, J.; Leftheriotis, G.; Abraham, P. Self-Reported Estimation of Usual Walking Speed Improves the Performance of Questionnaires Estimating Walking Capacity in Patients with Vascular-Type Claudication. J. Vasc. Surg. 2011, 54, 1360–1365. [Google Scholar] [CrossRef] [Green Version]
- Nordanstig, J.; Broeren, M.; Hensäter, M.; Perlander, A.; Österberg, K.; Jivegård, L. Six-Minute Walk Test Closely Correlates to “Real-Life” Outdoor Walking Capacity and Quality of Life in Patients with Intermittent Claudication. J. Vasc. Surg. 2014, 60, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Abraham, P.; Ouedraogo, N.; Tew, G.A.; Vielle, B.; Leftheriotis, G.; Mahe, G. Aging Reduces the Accuracy of Self-Reported Walking Limitation in Patients with Vascular-Type Claudication. J. Vasc. Surg. 2012, 56, 1025–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokkenrood, H.J.P.; Van Den Houten, M.M.L.; Houterman, S.; Breek, J.C.; Scheltinga, M.R.M.; Teijink, J.A.W. Agreements and Discrepancies between the Estimated Walking Distance, Nongraded and Graded Treadmill Testing, and Outside Walking in Patients with Intermittent Claudication. Ann. Vasc. Surg. 2015, 29, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Torino, C.; Manfredini, F.; Bolignano, D.; Aucella, F.; Baggetta, R.; Barillà, A.; Battaglia, Y.; Bertoli, S.; Bonanno, G.; Castellino, P.; et al. Physical Performance and Clinical Outcomes in Dialysis Patients: A Secondary Analysis of the Excite Trial EXCITE Working Group. Kidney Blood Press. Res. 2014, 39, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Khambati, H.; Boles, K.; Jetty, P. Google Maps Offers a New Way to Evaluate Claudication. J. Vasc. Surg. 2017, 65, 1467–1472. [Google Scholar] [CrossRef] [Green Version]
- Manfredini, F.; Malagoni, A.M.; Mascoli, F.; Mandini, S.; Taddia, M.C.; Basaglia, N.; Manfredini, R.; Conconi, F.; Zamboni, P. Training Rather than Walking—The Test in-Train out Program for Home-Based Rehabilitation in Peripheral Arteriopathy. Circ. J. 2008, 72, 946–952. [Google Scholar] [CrossRef] [Green Version]
- Manfredini, F.; Conconi, F.; Malagoni, A.M.; Manfredini, R.; Basaglia, N.; Mascoli, F.; Liboni, A.; Zamboni, P. Training Guided by Pain Threshold Speed. Effects of a Home-Based Program on Claudication. Int. Angiol. 2004, 23, 379–387. [Google Scholar]
- Manfredini, F.; Traina, L.; Gasbarro, V.; Straudi, S.; Caruso, L.; Fabbian, F.; Zamboni, P.; Manfredini, R.; Lamberti, N. Structured Pain-Free Exercise Progressively Improves Ankle-Brachial Index and Walking Ability in Patients with Claudication and Compressible Arteries: An Observational Study. Intern. Emerg. Med. 2021. [Google Scholar] [CrossRef]
- Gernigon, M.; Fouasson-Chailloux, A.; Colas-Ribas, C.; Noury-Desvaux, B.; Le Faucheur, A.; Abraham, P. Test-Retest Reliability of GPS Derived Measurements in Patients with Claudication. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Tew, G.; Nawaz, S.; Zwierska, I.; Blagojevic, M.; Saxton, J. Physiological Predictors of Maximum Treadmill Walking Performance in Patients with Intermittent Claudication. Int. J. Sport. Med. 2009, 30, 467–472. [Google Scholar] [CrossRef]
- Bajwa, A.; Wesolowski, R.; Patel, A.; Saha, P.; Ludwinski, F.; Smith, A.; Nagel, E.; Modarai, B. Assessment of Tissue Perfusion in the Lower Limb Current Methods and Techniques under Development. Circ. Cardiovasc. Imaging 2014, 7, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Grassi, B.; Quaresima, V. Near-Infrared Spectroscopy and Skeletal Muscle Oxidative Function in Vivo in Health and Disease: A Review from an Exercise Physiology Perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredini, F.; Lamberti, N.; Rossi, T.; Mascoli, F.; Basaglia, N.; Zamboni, P. A Toe Flexion NIRS Assisted Test for Rapid Assessment of Foot Perfusion in Peripheral Arterial Disease: Feasibility, Validity, and Diagnostic Accuracy. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Malagoni, A.M.; Felisatti, M.; Mandini, S.; Mascoli, F.; Manfredini, R.; Basaglia, N.; Zamboni, P. A Dynamic Objective Evaluation of Peripheral Arterial Disease by Near-Infrared Spectroscopy. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Manfredini, F.; Malagoni, A.M.; Mandini, S.; Felisatti, M.; Mascoli, F.; Basaglia, N.; Manfredini, R.; Mikhailidis, D.P.; Zamboni, P. Near-Infrared Spectroscopy Assessment Following Exercise Training in Patients with Intermittent Claudication and in Untrained Healthy Participants. Vasc. Endovasc. Surg. 2012, 46, 315–324. [Google Scholar] [CrossRef]
- Montgomery, P.S.; Gardner, A.W. The Clinical Utility of a Six-Minute Walk Test in Peripheral Arterial Occlusive Disease Patients. J. Am. Geriatr. Soc. 1998, 46, 706–711. [Google Scholar] [CrossRef]
- Manfredini, F.; Conconi, F.; Malagoni, A.M.; Manfredini, R.; Mascoli, F.; Liboni, A.; Zamboni, P. Speed Rather than Distance: A Novel Graded Treadmill Test to Asssess Claudication. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Kruidenier, L.M.; Nicolaï, S.P.; Willigendael, E.M.; de Bie, R.A.; Prins, M.H.; Teijink, J.A. Functional Claudication Distance: A Reliable and Valid Measurement to Assess Functional Limitation in Patients with Intermittent Claudication. BMC Cardiovasc. Disord. 2009, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Treat-Jacobson, D.; McDermott, M.M.; Bronas, U.G.; Campia, U.; Collins, T.C.; Criqui, M.H.; Gardner, A.W.; Hiatt, W.R.; Regensteiner, J.G.; Rich, K. Optimal Exercise Programs for Patients with Peripheral Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, E10–E33. [Google Scholar] [CrossRef]
- Oka, R.K.; Sanders, M.G. The Impact of Type 2 Diabetes and Peripheral Arterial Disease on Quality of Life. J. Vasc. Nurs. 2005, 23, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Birkett, S.T.; Harwood, A.E.; Caldow, E.; Ibeggazene, S.; Ingle, L.; Pymer, S. A Systematic Review of Exercise Testing in Patients with Intermittent Claudication: A Focus on Test Standardisation and Reporting Quality in Randomised Controlled Trials of Exercise Interventions. PLoS ONE 2021, 16, e0249277. [Google Scholar] [CrossRef] [PubMed]
- Abouhamda, A.; Alturkstani, M.; Jan, Y. Lower Sensitivity of Ankle-Brachial Index Measurements among People Suffering with Diabetes-Associated Vascular Disorders: A Systematic Review. SAGE Open Med. 2019, 7, 205031211983503. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Analyzed (n = 289) | |
|---|---|
| Age (years) | 71 ± 9 |
| Body mass index (kgm−2) | 25 ± 6 |
| Males, n (%) | 225 (78) |
| Risk factors, n (%) | |
| Smoking | 254 (88) |
| Current smoking | 72 (25) |
| Hypertension | 248 (86) |
| Hyperlipidaemia | 208 (72) |
| Diabetes | 156 (54) |
| Chronic kidney disease | 52 (18) |
| Comorbidities, n (%) | |
| Coronary artery disease | 87 (30) |
| Cerebrovascular disease | 14 (5) |
| Osteoarticular disease | 75 (26) |
| Rheumatic diseases | 12 (4) |
| Chronic-obstructive pulmonary disease | 15 (5) |
| Age-adjusted Charlson Comorbidity Index | 6 ± 2 |
| Peripheral artery disease | |
| Disease duration (years) | 6 ± 5 |
| Lower limb revascularization | 86 (27) |
| ABI more affected limb | 0.63 ± 0.22 |
| ABI less affected limb | 0.83 ± 0.19 |
| SR-CD | 6-CD | 6-MWD | T-CD | T-MWD | |
|---|---|---|---|---|---|
| SR-CD | - | 0.319 <0.001 | 0.291 <0.001 | 0.304 <0.001 | 0.254 <0.001 |
| 6-CD | 0.319 <0.001 | - | 0.560 <0.001 | 0.592 <0.001 | 0.496 <0.001 |
| 6-MWD | 0.291 <0.001 | 0.560 <0.001 | - | 0.512 <0.001 | 0.689 <0.001 |
| T-CD | 0.304 <0.001 | 0.592 <0.001 | 0.512 <0.001 | - | 0.739 <0.001 |
| T-MWD | 0.254 <0.001 | 0.496 <0.001 | 0.689 <0.001 | 0.739 <0.001 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberti, N.; Caruso, L.; Piva, G.; Traina, L.; Ficarra, V.; Zamboni, P.; Gasbarro, V.; Manfredini, F. Beyond the Patient’s Report: Self-Reported, Subjective, Objective and Estimated Walking Disability in Patients with Peripheral Artery Disease. Diagnostics 2021, 11, 1991. https://doi.org/10.3390/diagnostics11111991
Lamberti N, Caruso L, Piva G, Traina L, Ficarra V, Zamboni P, Gasbarro V, Manfredini F. Beyond the Patient’s Report: Self-Reported, Subjective, Objective and Estimated Walking Disability in Patients with Peripheral Artery Disease. Diagnostics. 2021; 11(11):1991. https://doi.org/10.3390/diagnostics11111991
Chicago/Turabian StyleLamberti, Nicola, Lorenzo Caruso, Giovanni Piva, Luca Traina, Valentina Ficarra, Paolo Zamboni, Vincenzo Gasbarro, and Fabio Manfredini. 2021. "Beyond the Patient’s Report: Self-Reported, Subjective, Objective and Estimated Walking Disability in Patients with Peripheral Artery Disease" Diagnostics 11, no. 11: 1991. https://doi.org/10.3390/diagnostics11111991
APA StyleLamberti, N., Caruso, L., Piva, G., Traina, L., Ficarra, V., Zamboni, P., Gasbarro, V., & Manfredini, F. (2021). Beyond the Patient’s Report: Self-Reported, Subjective, Objective and Estimated Walking Disability in Patients with Peripheral Artery Disease. Diagnostics, 11(11), 1991. https://doi.org/10.3390/diagnostics11111991









