Current Status of Photodynamic Diagnosis for Gastric Tumors
Abstract
:1. Introduction
2. Basic of Photodynamic Endoscopic Diagnosis
2.1. Photosensitizer
2.2. Light Source
3. Photodynamic Diagnosis in the Other Fields
4. Photodynamic Endoscopic Diagnosis of Gastric Tumors
5. PDED for Gastric Tumors at Our Hospital
6. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Shikuwa, S.; Yamaguchi, N.; Fukuda, E.; Ikeda, K.; Nishiyama, H.; Ohnita, K.; Mizuta, Y.; Shiozawa, J.; Kohno, S. Endoscopic submucosal dissection for early gastric cancer: A large-scale feasibility study. Gut 2009, 58, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, K.; Ono, H.; Hasuike, N.; Takashima, A.; Minashi, K.; Boku, N.; Kushima, R.; Katayama, H.; Ogawa, G.; Fukuda, H.; et al. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer 2021, 24, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Hasuike, N.; Ono, H.; Boku, N.; Mizusawa, J.; Takizawa, K.; Fukuda, H.; Oda, I.; Doyama, H.; Kaneko, K.; Hori, S.; et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): The Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer 2018, 21, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Ezoe, Y.; Muto, M.; Uedo, N.; Doyama, H.; Yao, K.; Oda, I.; Kaneko, K.; Kawahara, Y.; Yokoi, C.; Sugiura, Y.; et al. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology 2011, 141, 2017–2025. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Doyama, H.; Gotoda, T.; Ishikawa, H.; Nagahama, T.; Yokoi, C.; Oda, I.; Machida, H.; Uchita, K.; Tabuchi, M. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: A prospective multicenter feasibility study. Gastric Cancer 2014, 17, 669–679. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, M.; Esposito, G.; Libânio, D.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Image-enhanced endoscopy for gastric preneoplastic conditions and neoplastic lesions: A systematic review and meta-analysis. Endoscopy 2020, 52, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Wang, L.; Zhang, L.; Chen, P.; Xu, B.; Peng, D.; Yang, M.; Tan, Y.; Cai, C.; Li, H.; et al. Magnifying endoscopy in detecting early gastric cancer. Medicine 2021, 100, e23934. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, H.; Fan, C.; Chen, S.; Liu, A. Comparison of the diagnostic efficacy of blue laser imaging with narrow band imaging for gastric cancer and precancerous lesions: A meta-analysis. Rev. Esp. Enferm. Dig. 2020, 12, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Yao, K.; Kaise, M.; Kato, M.; Uedo, N.; Yagi, K.; Tajiri, H. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig. Endosc. 2016, 28, 379–393. [Google Scholar] [CrossRef] [Green Version]
- Akazawa, Y.; Ueyama, H.; Yao, T.; Komori, H.; Takeda, T.; Matsumoto, K.; Matsumoto, K.; Asaoka, D.; Hojo, M.; Watanabe, S.; et al. Usefulness of demarcation of differentiated-type early gastric cancers after Helicobacter pylori eradication by magnifying endoscopy with narrow-band imaging. Digestion 2018, 98, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Anagnostopoulos, G.K.; Ragunath, K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy 2009, 41, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N.; Doyama, H.; Yano, T.; Horimatsu, T.; Uedo, N.; Yamamoto, Y.; Kakushima, N.; Kanzaki, H.; Hori, S.; Yao, K.; et al. Early gastric cancer detection in high-risk patients: A multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut 2021, 70, 67–75. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hashimoto, S.; Nishikura, K.; Mizuno, K.I.; Takeuchi, M.; Sato, Y.; Ajioka, Y.; Aoyagi, Y. Magnifying narrow-band imaging of surface maturation in early differentiated-type gastric cancers after Helicobacter pylori eradication. J. Gastroenterol. 2013, 48, 1332–1342. [Google Scholar] [CrossRef]
- Saka, A.; Yagi, K.; Nimura, S. Endoscopic and histological features of gastric cancers after successful Helicobacter pylori eradication therapy. Gastric Cancer 2016, 19, 524–530. [Google Scholar] [CrossRef]
- Horiguchi, N.; Tahara, T.; Kawamura, T.; Okubo, M.; Tahara, S.; Nagasaka, M.; Nakagawa, Y.; Shibata, T.; Ohmiya, N. A comparative study of white light endoscopy, chromoendoscopy and magnifying endoscopy with narrow band imaging in the diagnosis of early gastric cancer after Helicobacter pylori eradication. J. Gastrointestin. Liver Dis. 2017, 26, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. The use of a derivative of hematoporhyrin in tumor detection. J. Natl. Cancer Inst. 1961, 26, 1–11. [Google Scholar] [PubMed]
- Dougherty, T.J.; Lawrence, G.; Kaufman, J.H.; Boyle, D.; Weishaupt, K.R.; Goldfarb, A. Photoradiation in the treatment of recurrent breast carcinoma. J. Natl. Cancer Inst. 1979, 62, 231–237. [Google Scholar] [PubMed]
- Tsuchihashi, T.; Mori, K.; Ueyama, K.; Yoneya, S. Five-year results of photodynamic therapy with verteporfin for Japanese patients with neovascular age-related macular degeneration. Clin. Ophthalmol. 2013, 7, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, R.; Lee, J.; Yang, S. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yonemura, Y.; Endo, Y.; Canbay, E.; Liu, Y.; Ishibashi, H.; Mizumoto, A.; Hirano, M.; Imazato, Y.; Takao, N.; Ichinose, M.; et al. Photodynamic detection of peritoneal metastases using 5-aminolevulinic acid (ALA). Cancers 2017, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, F.S.; Bentley, M.V. Photodynamic therapy of skin cancers: Sensitizers, clinical studies and future directives. Pharm. Res. 2000, 17, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Rick, K.; Sroka, R.; Stepp, H.; Kriegmair, M.; Huber, R.M.; Jacob, K.; Baumgartner, R. Pharmacokinetics of 5-aminolevulinic acid-induced protoporphyrin IX in skin and blood. J. Photochem. Photobiol. B. 1997, 40, 313–319. [Google Scholar] [CrossRef]
- Knap, B.; Przystupski, D.; Saczko, J.; Ewa, K.; Knap-Czop, K.; Kotli, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet. Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Stummer, W.; Tonn, J.C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pietsch, T.; Pichlmeier, U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 2014, 74, 310–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, D.W.; Valdes, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, X.F.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez Valle, R.; Tejada Solis, S.; Idoate Gastearena, M.A.; de Eulate, R.G.; Domínguez Echávarri, P.; Aristu Mendiroz, J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: Volumetric analysis of extent of resection in single-center experience. J. Neurooncol. 2011, 102, 105–113. [Google Scholar] [CrossRef]
- Golub, D.; Hyde, J.; Dogra, S.; Nicholson, J.; Kirkwood, K.A.; Gohel, P.; Loftus, S.; Schwartz, T.H. Intraoperative MRI versus 5-ALA in high-grade glioma resection: A network meta-analysis. J. Neurosurg. 2021, 134, 484–498. [Google Scholar] [CrossRef]
- Hadjipanayis, C.; Stummer, W. 5-ALA and FDA Approved for Glimoa Surgery. Physiol. Behav. 2017, 141, 479–486. [Google Scholar] [CrossRef]
- Rall, D.P.; Loo, T.L.; Lane, M.; Kelly, M.G. Appearance and persistence of fluorescent material in tumor tissue after tetracycline administration. J. Natl. Cancer Inst. 1957, 19, 79–85. [Google Scholar] [PubMed]
- Whitmore, W.F.J.; Bush, I.M.; Esquivel, E. Tetracycline ultraviolet fluorescence in bladder carcinoma. Cancer 1964, 17, 1528–1532. [Google Scholar] [CrossRef]
- Karl, A.; Tritschler, S.; Stanislaus, P.; Gratzke, C.; Tilki, D.; Strittmatter, F.; Knüchel, R.; Stief, C.G.; Zaak, D. Positive urine cytology but negative white-light cystoscopy: An indication for fluorescence cystoscopy? BJU Int. 2009, 103, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Denzinger, S.; Burger, M.; Walter, B.; Knuechel, R.; Roessler, W.; Wieland, W.F.; Filbeck, T. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 2007, 69, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Hungerhuber, E.; Stepp, H.; Kriegmair, M.; Stief, C.; Hofstetter, A.; Hartmann, A.; Knuechel, R.; Karl, A.; Tritschler, S.; Zaak, D. Seven years’ experience with 5-aminolevulinic acid in detection of transitional cell carcinoma of the bladder. Urology 2007, 69, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fukuhara, H.; Shimamoto, T.; Kamada, M.; Iiyama, T.; Miyamura, M.; Kurabayashi, A.; Furihata, M.; Tanimura, M.; Watanabe, H.; et al. Comparison between intravesical and oral administration of 5-aminolevulinic acid in the clinical benefit of photodynamic diagnosis for nonmuscle invasive bladder cancer. Cancer 2012, 118, 1062–1074. [Google Scholar] [CrossRef]
- Inoue, K.; Anai, S.; Fujimoto, K.; Hirao, Y.; Furuse, H.; Kai, F.; Ozono, S.; Hara, T.; Matsuyama, H.; Oyama, M.; et al. Oral 5-aminolevulinic acid mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A randomized, double-blind, multicentre phase II/III study. Photodiagn. Photodyn. Ther. 2015, 12, 193–200. [Google Scholar] [CrossRef]
- Shen, P.; Yang, J.; Wei, W.; Li, Y.; Li, D.; Zeng, H.; Wang, J. Effects of fluorescent light-guided transurethral resection on non-muscle-invasive bladder cancer: A systematic review and meta-analysis. BJU Int. 2012, 110, E209–E215. [Google Scholar] [CrossRef]
- Grossman, H.B.; Gomella, L.; Fradet, Y.; Morales, A.; Presti, J.; Ritenour, C.; Nseyo, U.; Droller, M.J. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J. Urol. 2007, 178, 62–67. [Google Scholar] [CrossRef]
- Geavlete, B.; Multescu, R.; Georgescu, D.; Jecu, M.; Stanescu, F.; Geavlete, P. Treatment changes and long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: Does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU Int. 2012, 109, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Geavlete, B.; Jecu, M.; Multescu, R.; Georgescu, D.; Geavlete, P. HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer—Re-TURBT recurrence rates in a prospective, randomized study. Urology 2010, 76, 664–669. [Google Scholar] [CrossRef]
- Nakai, Y.; Inoue, K.; Tsuzuki, T.; Shimamoto, T.; Shuin, T.; Nagao, K.; Matsuyama, H.; Oyama, M.; Furuse, H.; Ozono, S.; et al. Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A multicenter phase III study. Int. J. Urol. 2018, 25, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denzinger, S.; Wieland, W.F.; Otto, W.; Filbeck, T.; Knuechel, R.; Burger, M. Does photodynamic transurethral resection of bladder tumour improve the outcome of initial T1 high-grade bladder cancer? A long-term follow-up of a randomized study. BJU Int. 2008, 101, 566–569. [Google Scholar] [CrossRef]
- Mowatt, G.; N’Dow, J.; Vale, L.; Nabi, G.; Boachie, C.; Cook, J.A.; Fraser, C.; Griffiths, T.R.L. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: Systematic review and meta-analysis. Int. J. Technol. Assess. Health Care 2011, 27, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, M.; Grossman, H.B.; Droller, M.; Schmidbauer, J.; Hermann, G.; Drăgoescu, O.; Ray, E.; Fradet, Y.; Karl, A.; Burgués, J.P.; et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis of detection and recurrence based on raw data. Eur. Urol. 2013, 64, 846–854. [Google Scholar] [CrossRef]
- Kausch, I.; Sommerauer, M.; Montorsi, F.; Stenzl, A.; Jacqmin, D.; Jichlinski, P.; Jocham, D.; Ziegler, A.; Vonthein, R. Photodynamic diagnosis in non-muscle-invasive bladder cancer: A systematic review and cumulative analysis of prospective studies. Eur. Urol. 2010, 57, 595–606. [Google Scholar] [CrossRef]
- Kim, H.I.; Wilson, B.C. Photodynamic diagnosis and therapy for peritoneal carcinomatosis from gastrointestinal cancers: Status, opportunities, and challenges. J. Gastric Cancer 2020, 20, 355–375. [Google Scholar] [CrossRef]
- Löning, M.; Diddens, H.; Küpker, W.; Diedrich, K.; Hüttmann, G. Laparoscopic fluorescence detection of ovarian carcinoma metastases using 5-aminolevulinic acid-induced protoporphyrin IX. Cancer 2004, 100, 1650–1656. [Google Scholar] [CrossRef]
- Zöpf, T.; Schneider, A.R.J.; Weickert, U.; Riemann, J.F.; Arnold, J.C. Improved preoperative tumor staging by 5-aminolevulinic acid induced fluorescence laparoscopy. Gastrointest. Endosc. 2005, 62, 763–767. [Google Scholar] [CrossRef]
- Kishi, K.; Fujiwara, Y.; Yano, M.; Motoori, M.; Sugimura, K.; Ohue, M.; Noura, S.; Marubashi, S.; Takahashi, H.; Sakon, M. Diagnostic laparoscopy with 5-aminolevulinic-acid-mediated photodynamic diagnosis enhances the detection of peritoneal micrometastases in advanced gastric cancer. Oncology 2014, 87, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kishi, K.; Fujiwara, Y.; Yano, M.; Motoori, M.; Sugimura, K.; Takahashi, H.; Ohue, M.; Sakon, M. Usefulness of diagnostic laparoscopy with 5-aminolevulinic acid (ALA)-mediated photodynamic diagnosis for the detection of peritoneal micrometastasis in advanced gastric cancer after chemotherapy. Surg. Today 2016, 46, 1427–1434. [Google Scholar] [CrossRef]
- Ushimaru, Y.; Fujiwara, Y.; Kishi, K.; Sugimura, K.; Omori, T.; Moon, J.-H.; Yanagimoto, Y.; Ohue, M.; Yasui, M.; Takahashi, H.; et al. Prognostic significance of basing treatment strategy on the results of photodynamic diagnosis in advanced gastric cancer. Ann. Surg. Oncol. 2017, 24, 983–989. [Google Scholar] [CrossRef]
- Mayinger, B.; Reh, H.; Hahn, E.G. Endoscopic photodynamic diagnosis: Oral aminolevulinic acid is a marker of GI cancer and dysplastic lesions. Gastrointest. Endosc. 1999, 50, 242–246. [Google Scholar] [CrossRef]
- Nakamura, T.; Oinuma, T. Usefulness of photodynamic diagnosis and therapy using talaporfin sodium for an advanced-aged patient with inoperable gastric cancer (a secondary publication). Laser Ther. 2014, 23, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Nishikawa, J.; Hamabe, K.; Goto, A.; Nishimura, J.; Shibata, H.; Nagao, M.; Sasaki, S.; Hashimoto, S.; Okamoto, T.; et al. Preliminary study of photodynamic diagnosis using 5-aminolevulinic acid in gastric and colorectal tumors. World J. Gastroenterol. 2015, 21, 6706–6712. [Google Scholar] [CrossRef]
- Ogihara, K.; Isomoto, H.; Kurumi, H.; Kanda, T.; Hashisako, M.; Tabata, K.; Ishii, H.; Ohnita, K.; Yamaguchi, N.; Akazawa, Y.; et al. Expression of coproporphyrinogen oxidase is associated with detection of upper gastrointestinal carcinomas by 5-aminolevulinic acid-mediated photodynamic diagnosis. Photodiagn. Photodyn. Ther. 2017, 19, 15–21. [Google Scholar] [CrossRef]
- Kurumi, H.; Kanda, T.; Kawaguchi, K.; Yashima, K.; Koda, H.; Ogihara, K.; Matsushima, K.; Nakao, K.; Saito, H.; Fujiwara, Y.; et al. Protoporphyrinogen oxidase is involved in the fluorescence intensity of 5-aminolevulinic acid-mediated laser-based photodynamic endoscopic diagnosis for early gastric cancer. Photodiagn. Photodyn. Ther. 2018, 22, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Kinoshita, H.; Ikebuchi, Y.; Kanda, T.; Yamashita, T.; Kurumi, H.; Fujii, M.; Edano, M.; Hasegawa, T.; Onoyama, T.; et al. Next-generation laser-based photodynamic endoscopic diagnosis using 5-aminolevulinic acid for early gastric adenocarcinoma and gastric adenoma. Ann. Gastroenterol. 2020, 33, 257–264. [Google Scholar] [CrossRef]
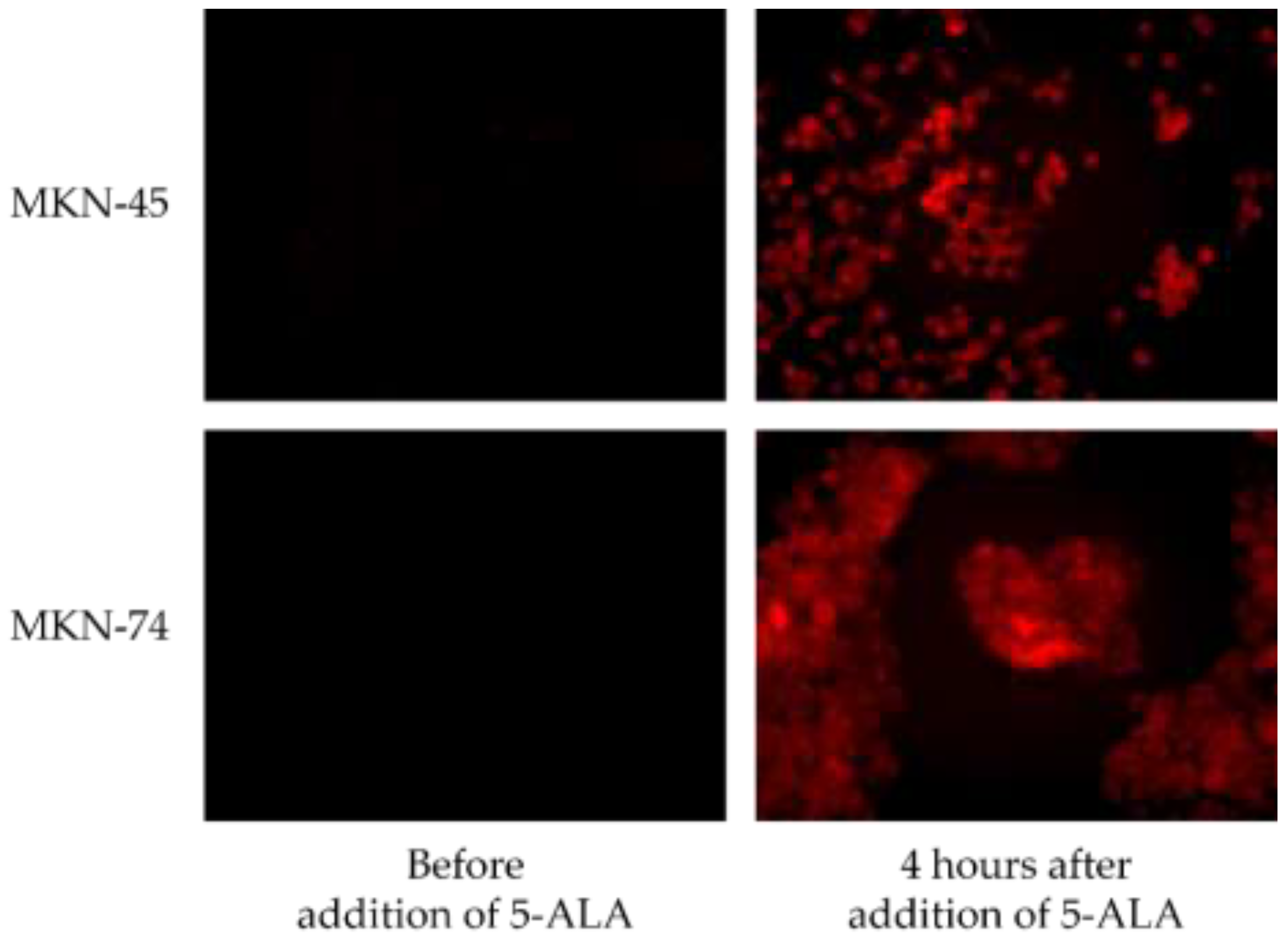

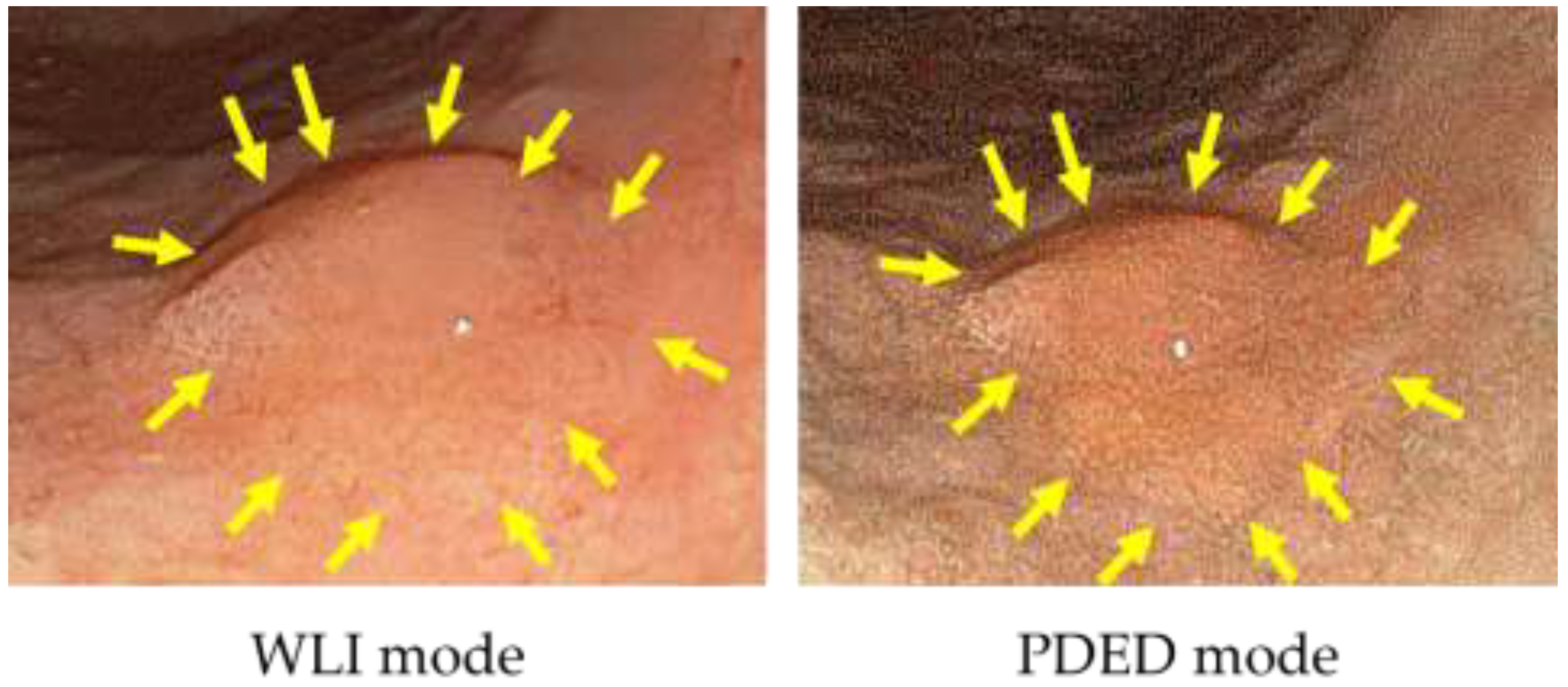
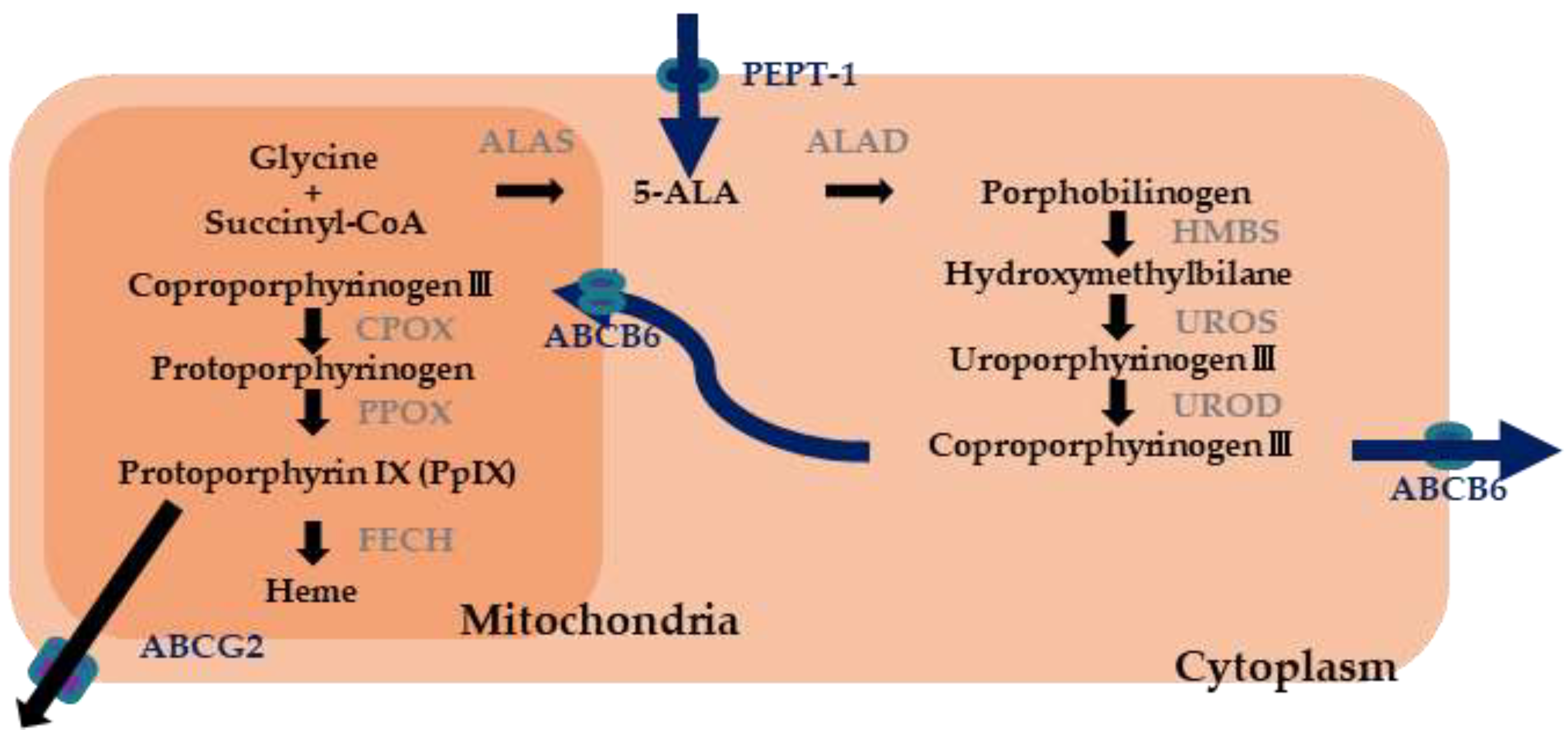


| PDED Positive (n = 42) | PDED Negative (n = 7) | Univariate Analysis | Multivariate Analysis | |
|---|---|---|---|---|
| Sex (male/female) | 28/8 | 4/3 | 0.87 * | |
| Age (median) | 74.5 | 64 | 0.42 ** | |
| Site of tumor (Upper/middle/lower) | 4/25/13 | 0/4/3 | 0.25 * | |
| Macroscopic (elevated/flat · depressed) | 22/20 | 0/7 | 0.01 * | 0.42 |
| Tumor diameter (median) | 25 | 7 | <0.01 ** | 0.06 |
| Invasion depth (intramucosal/submucosal) | 38/4 | 6/1 | 0.75 * | |
| Histopathology (tub 1)/sig 2)/adenoma) | 33/0/9 | 3/4/0 | <0.01 * | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurumi, H.; Kanda, T.; Ikebuchi, Y.; Yoshida, A.; Kawaguchi, K.; Yashima, K.; Isomoto, H. Current Status of Photodynamic Diagnosis for Gastric Tumors. Diagnostics 2021, 11, 1967. https://doi.org/10.3390/diagnostics11111967
Kurumi H, Kanda T, Ikebuchi Y, Yoshida A, Kawaguchi K, Yashima K, Isomoto H. Current Status of Photodynamic Diagnosis for Gastric Tumors. Diagnostics. 2021; 11(11):1967. https://doi.org/10.3390/diagnostics11111967
Chicago/Turabian StyleKurumi, Hiroki, Tsutomu Kanda, Yuichiro Ikebuchi, Akira Yoshida, Koichiro Kawaguchi, Kazuo Yashima, and Hajime Isomoto. 2021. "Current Status of Photodynamic Diagnosis for Gastric Tumors" Diagnostics 11, no. 11: 1967. https://doi.org/10.3390/diagnostics11111967
APA StyleKurumi, H., Kanda, T., Ikebuchi, Y., Yoshida, A., Kawaguchi, K., Yashima, K., & Isomoto, H. (2021). Current Status of Photodynamic Diagnosis for Gastric Tumors. Diagnostics, 11(11), 1967. https://doi.org/10.3390/diagnostics11111967








