Texture and Color Enhancement Imaging Increases Color Changes and Improves Visibility for Squamous Cell Carcinoma Suspicious Lesions in the Pharynx and Esophagus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Endoscopy System and Setting
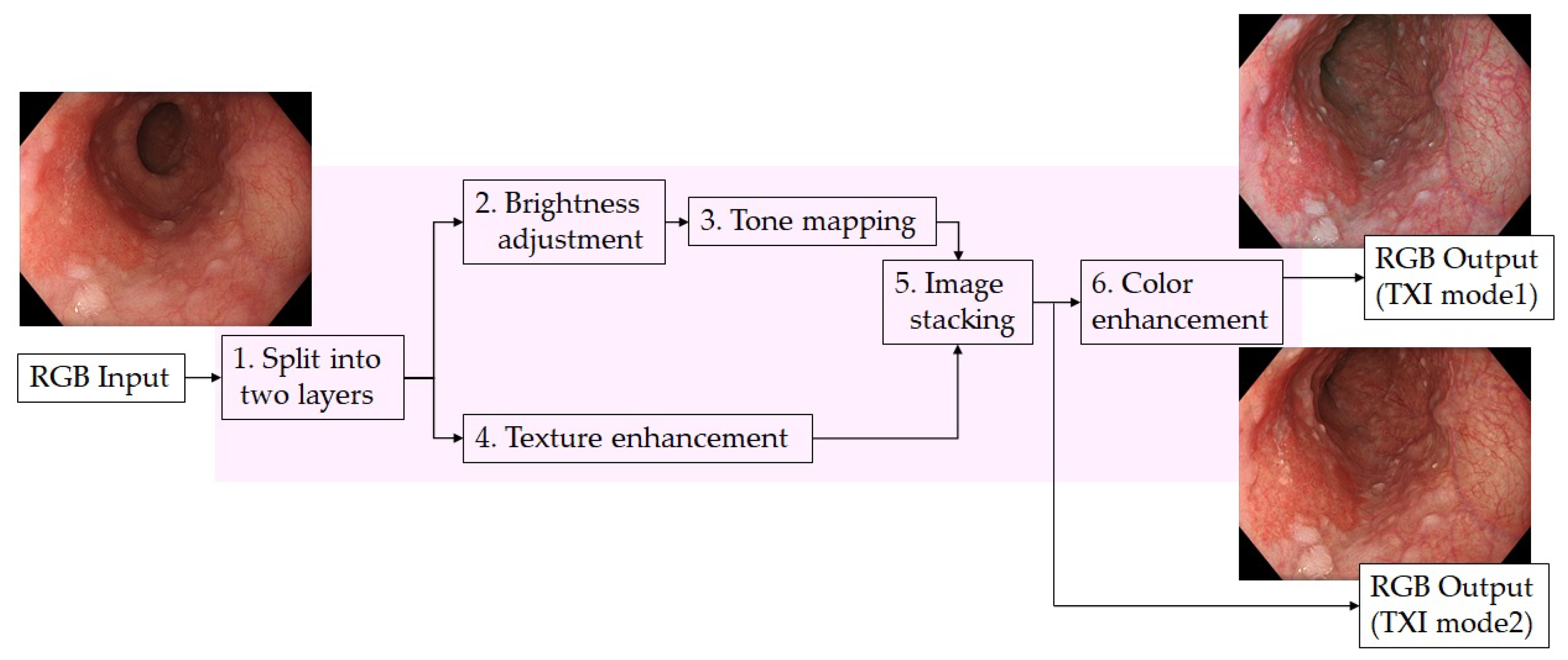
2.3. Texture and Color Enhancement Imaging (TXI)
2.4. Endoscopic Procedure and Collecting Endoscopic Images
2.5. Color Analysis
2.6. Outcomes
2.7. Pathologic Diagnosis
2.8. The Evaluation of Endoscopic Images
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Yamashina, T.; Ishihara, R.; Nagai, K.; Matsuura, N.; Matsui, F.; Ito, T.; Fujii, M.; Yamamoto, S.; Hanaoka, N.; Takeuchi, Y.; et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am. J. Gastroenterol. 2013, 108, 544–551. [Google Scholar] [CrossRef]
- Takahashi, K.; Hashimoto, S.; Mizuno, K.I.; Kobayashi, T.; Tominaga, K.; Sato, H.; Kohisa, J.; Ikarashi, S.; Hayashi, K.; Takeuchi, M.; et al. Management decision based on lymphovascular involvement leads to favorable outcomes after endoscopic treatment of esophageal squamous cell carcinoma. Endoscopy 2018, 50, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Minashi, K.; Yano, T.; Saito, Y.; Oda, I.; Nonaka, S.; Omori, T.; Sugiura, H.; Goda, K.; Kaise, M.; et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: A multicenter randomized controlled trial. J. Clin. Oncol. 2010, 8, 1566–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goda, K.; Dobashi, A.; Tajiri, H. Perspectives on narrow-band imaging endoscopy for superficial squamous neoplasms of the orohypopharynx and esophagus. Dig. Endosc. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Tajiri, H.; Niwa, H. Proposal for a consensus terminology in endoscopy: How should different endoscopic imaging techniques be grouped and defined? Endoscopy 2008, 40, 775–778. [Google Scholar] [CrossRef]
- Kobayashi, K.; Miyahara, R.; Funasaka, K.; Furukawa, K.; Sawada, T.; Maeda, K.; Yamamura, T.; Ishikawa, T.; Ohno, E.; Nakamura, M.; et al. Color information from linked color imaging is associated with invasion depth and vascular diameter in superficial esophageal squamous cell carcinoma. Dig. Endosc. 2020, 32, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Dobashi, A.; Goda, K.; Furuhashi, H.; Matsui, H.; Hara, Y.; Kamba, S.; Kobayashi, M.; Sumiyama, K.; Hirooka, S.; Hamatani, S.; et al. Diagnostic efficacy of dual-focus endoscopy with narrow-band imaging using simplified dyad criteria for superficial esophageal squamous cell carcinoma. J. Gastroenterol. 2019, 54, 501–510. [Google Scholar] [CrossRef]
- Diao, W.; Huang, X.; Shen, L.; Zeng, Z. Diagnostic ability of blue laser imaging combined with magnifying endoscopy for early esophageal cancer. Dig. Liver Dis. 2018, 50, 1035–1040. [Google Scholar] [CrossRef]
- Nakamura, K.; Urabe, Y.; Oka, S.; Nagasaki, N.; Yorita, N.; Hata, K.; Masuda, K.; Kurihara, M.; Kotachi, T.; Boda, T.; et al. Usefulness of linked color imaging in the early detection of superficial esophageal squamous cell carcinomas. Esophagus 2021, 18, 118–124. [Google Scholar] [CrossRef]
- Goda, K.; Dobashi, A.; Yoshimura, N.; Aihara, H.; Kato, M.; Sumiyama, K.; Toyoizumi, H.; Kato, T.; Saijo, H.; Ikegami, M.; et al. Dual-focus versus conventional magnification endoscopy for the diagnosis of superficial squamous neoplasms in the pharynx and esophagus: A randomized trial. Endoscopy 2016, 48, 321–329. [Google Scholar] [CrossRef]
- Morita, F.H.; Bernardo, W.M.; Ide, E.; Rocha, R.S.P.; Aquino, J.C.M.; Minata, M.K.; Yamazaki, K.; Marques, S.B.; Sakai, P.; de Moura, E.G.H. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: A systematic review and meta-analysis. BMC Cancer 2017, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sato, T. TXI: Texture and Color Enhancement Imaging for Endoscopic Image Enhancement. J. Healthc Eng. 2021, 2021, 5518948. [Google Scholar] [CrossRef]
- Ishikawa, T.; Matsumura, T.; Okimoto, K.; Nagashima, A.; Shiratori, W.; Kaneko, T.; Oura, H.; Tokunaga, M.; Akizue, N.; Ohta, Y.; et al. Efficacy of Texture and Color Enhancement Imaging in visualizing gastric mucosal atrophy and gastric neoplasms. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Kuehni, R.G. Color-tolerance data and the tentative CIE 1976 L a b formula. J. Opt. Soc. Am. 1976, 66, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Takenaka, R.; Kawahara, Y.; Kawai, D.; Obayashi, Y.; Baba, Y.; Sakae, H.; Gotoda, T.; Kono, Y.; Miura, K.; et al. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc Int. Open. 2017, 5, E1005–E1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Japanese Esophageal Society. Japanese classification of esophageal cancer: Parts II and III. Esophagus. 2009, 6, 71–94. [Google Scholar] [CrossRef]
- Yoshida, T.; Inoue, H.; Usui, S.; Satodate, H.; Fukami, N.; Kudo, S.E. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004, 59, 288–295. [Google Scholar] [CrossRef]
- Muto, M.; Nakane, M.; Katada, C.; Sano, Y.; Ohtsu, A.; Esumi, H.; Ebihara, S.; Yoshida, S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer 2004, 101, 1375–1381. [Google Scholar] [CrossRef]
- Ishihara, R.; Takeuchi, Y.; Chatani, R.; Kidu, T.; Inoue, T.; Hanaoka, N.; Yamamoto, S.; Higashino, K.; Uedo, N.; Iishi, H.; et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis. Esophagus. 2010, 23, 480–486. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | N | Rate | |
|---|---|---|---|
| Location | Pharynx | 15 | 25.4% |
| Esophagus | 44 | 74.6% | |
| Histology | SCC | 24 | 40.7% |
| HGIN | 11 | 18.6% | |
| LGIN | 16 | 27.1% | |
| Indefinite for neoplasia, no neoplasia | 8 | 13.6% | |
| Type | IIb | 24 | 40.7% |
| IIa or IIc | 35 | 59.3% | |
| Size | <10 mm | 27 | 45.8% |
| ≥10 mm | 32 | 54.2% | |
| WLI | TXI Mode1 | TXI Mode2 | NBI | |
|---|---|---|---|---|
| ΔL | 6.2(4.4) | 8.7 (5.4) | 8.1 (5.1) | 13.2 (7.6) |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 | |
| Δa | 5.7 (3.7) | 10.8 (5.4) | 7.2 (3.9) | 6.9 (4.5) |
| p (vs. WLI )* | <0.001 | <0.001 | <0.001 | |
| Δb | 4.8 (3.4) | 8.3 (4.8) | 5.6 (3.4) | 1.8 (1.3) |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 | |
| ΔE | 11.6 (4.8) | 18.6 (6.8) | 14.3 (5.5) | 17.2 (7.3) |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 |
| WLI | TXI Mode1 | TXI Mode2 | NBI | ||
|---|---|---|---|---|---|
| Location | Pharynx | 10.4(3.8) | 16.2(5.6) | 13.3(4.8) | 13.8(5.9) |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 | ||
| Esophagus | 11.9(5.1) | 19.4(7.0) | 14.7(5.8) | 18.4(7.4) | |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 | ||
| Histology | SCC | 12.6(5.4) | 19.8(5.6) | 15.2(4.9) | 19.2(7.1) |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 | ||
| HGIN | 11.4(3.1) | 19.6(7.2) | 14.7(2.7) | 15.4(4.5) | |
| p (vs. WLI) * | 0.001 | 0.001 | 0.001 | ||
| LGIN | 10.0(3.4) | 16.0(5.7) | 12.1(4.4) | 14.7(5.4) | |
| p (vs. WLI) * | 0.001 | 0.001 | 0.001 | ||
| Indefinite for neoplasia, no neoplasia | 11.6(6.8) | 18.6(10.8) | 15.7(10.3) | 18.8(12.0) | |
| p (vs. WLI) * | 0.008 | 0.008 | 0.008 | ||
| Type | IIb | 10.6(4.4) | 18.4(6.8) | 14.2(5.0) | 17.1(7.3) |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 | ||
| IIa or IIc | 12.9(5.1) | 18.9(6.9) | 14.6(6.4) | 17.4(7.4) | |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 | ||
| Size | <10 mm | 11.0(4.3) | 18.6(8.0) | 14.5(6.3) | 17.6(7.6) |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 | ||
| ≥10 mm | 12.2(5.3) | 18.5(5.1) | 14.2(4.6) | 16.7(7.0) | |
| p (vs. WLI) * | <0.001 | <0.001 | <0.001 |
| Experts | Non-Experts | p | ||||
|---|---|---|---|---|---|---|
| TXI mode1 visibility | Score, mean (SD) * | 0.68 (0.34) | 0.58 (0.33) | 0.072 | ||
| Number reporting an improvement | ||||||
| 0 | 6 | 10.2% | 6 | 10.2% | 0.229 | |
| 1 | 11 | 18.6% | 17 | 28.8% | ||
| 2 | 18 | 30.5% | 22 | 37.3% | ||
| 3 | 24 | 40.7% | 14 | 23.7% | ||
| Number reporting a decline | ||||||
| 0 | 59 | 100.0% | 59 | 100.0% | - | |
| 1 | 0 | 0.0% | 0 | 0.0% | ||
| 2 | 0 | 0.0% | 0 | 0.0% | ||
| 3 | 0 | 0.0% | 0 | 0.0% | ||
| TXI mode2 visibility | Score, mean (SD) * | 0.49 (0.37) | 0.31 (0.35) | <0.001 | ||
| Number reporting an improvement | ||||||
| 0 | 10 | 16.9% | 23 | 39.0% | 0.022 | |
| 1 | 20 | 33.9% | 21 | 35.6% | ||
| 2 | 16 | 27.1% | 8 | 13.6% | ||
| 3 | 13 | 22.0% | 7 | 11.9% | ||
| Number reporting a decline | ||||||
| 0 | 56 | 94.9% | 57 | 96.6% | 1.000 | |
| 1 | 3 | 5.1% | 2 | 3.4% | ||
| 2 | 0 | 0.0% | 0 | 0.0% | ||
| 3 | 0 | 0.0% | 0 | 0.0% | ||
| NBI visibility | Score, mean (SD) * | 0.92 (0.17) | 0.75 (0.31) | <0.001 | ||
| Number reporting an improvement | ||||||
| 0 | 0 | 0.0% | 2 | 3.4% | 0.005 | |
| 1 | 2 | 3.4% | 10 | 16.9% | ||
| 2 | 12 | 20.3% | 18 | 30.5% | ||
| 3 | 45 | 76.3% | 29 | 49.2% | ||
| Number reporting a decline | ||||||
| 0 | 59 | 100.0% | 57 | 96.6% | 0.496 | |
| 1 | 0 | 0.0% | 2 | 3.4% | ||
| 2 | 0 | 0.0% | 0 | 0.0% | ||
| 3 | 0 | 0.0% | 0 | 0.0% | ||
| Experts | Non-Experts | ||||||
|---|---|---|---|---|---|---|---|
| TXI mode1 visibility | TXI mode2 visibility | NBI visibility | TXI mode1 visibility | TXI mode2 visibility | NBI visibility | ||
| Location | Pharynx | 0.68(0.29) | 0.48(0.34) | 0.92(0.14) | 0.51(0.32) | 0.23(0.29) | 0.63(0.32) |
| Esophagus | 0.68(0.36) | 0.50(0.38) | 0.91(0.18) | 0.61(0.33) | 0.34(0.37) | 0.79(0.31) | |
| p | 0.962 | 0.857 | 0.901 | 0.329 | 0.330 | 0.089 | |
| Histology | SCC | 0.77(0.34) | 0.54(0.38) | 0.93(0.13) | 0.68(0.29) | 0.34(0.38) | 0.83(0.24) |
| HGIN | 0.67(0.34) | 0.63(0.33) | 0.97(0.09) | 0.57(0.35) | 0.39(0.42) | 0.67(0.27) | |
| LGIN | 0.72(0.30) | 0.44(0.39) | 0.94(0.19) | 0.50(0.31) | 0.24(0.28) | 0.82(0.30) | |
| No neoplasia, indefinite for neoplasia | 0.33(0.27) | 0.29(0.29) | 0.76(0.24) | 0.50(0.41) | 0.25(0.31) | 0.46(0.44) | |
| p | 0.013 | 0.202 | 0.036 | 0.328 | 0.648 | 0.019 | |
| Type | IIb | 0.77(0.26) | 0.53(0.34) | 0.92(0.17) | 0.64(0.32) | 0.35(0.33) | 0.73(0.31) |
| IIa or IIc | 0.61(0.38) | 0.47(0.39) | 0.91(0.17) | 0.55(0.33) | 0.28(0.37) | 0.76(0.32) | |
| p | 0.060 | 0.644 | 0.835 | 0.306 | 0.475 | 0.678 | |
| Size | <10 mm | 0.73(0.33) | 0.50(0.37) | 0.91(0.14) | 0.66(0.31) | 0.30(0.25) | 0.82(0.23) |
| ≥10 mm | 0.63(0.35) | 0.49(0.37) | 0.92(0.19) | 0.52(0.33) | 0.32(0.36) | 0.68(0.36) | |
| p | 0.244 | 0.923 | 0.864 | 0.090 | 0.898 | 0.076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobashi, A.; Ono, S.; Furuhashi, H.; Futakuchi, T.; Tamai, N.; Yamauchi, T.; Suka, M.; Sumiyama, K. Texture and Color Enhancement Imaging Increases Color Changes and Improves Visibility for Squamous Cell Carcinoma Suspicious Lesions in the Pharynx and Esophagus. Diagnostics 2021, 11, 1971. https://doi.org/10.3390/diagnostics11111971
Dobashi A, Ono S, Furuhashi H, Futakuchi T, Tamai N, Yamauchi T, Suka M, Sumiyama K. Texture and Color Enhancement Imaging Increases Color Changes and Improves Visibility for Squamous Cell Carcinoma Suspicious Lesions in the Pharynx and Esophagus. Diagnostics. 2021; 11(11):1971. https://doi.org/10.3390/diagnostics11111971
Chicago/Turabian StyleDobashi, Akira, Shingo Ono, Hiroto Furuhashi, Toshiki Futakuchi, Naoto Tamai, Takashi Yamauchi, Machi Suka, and Kazuki Sumiyama. 2021. "Texture and Color Enhancement Imaging Increases Color Changes and Improves Visibility for Squamous Cell Carcinoma Suspicious Lesions in the Pharynx and Esophagus" Diagnostics 11, no. 11: 1971. https://doi.org/10.3390/diagnostics11111971
APA StyleDobashi, A., Ono, S., Furuhashi, H., Futakuchi, T., Tamai, N., Yamauchi, T., Suka, M., & Sumiyama, K. (2021). Texture and Color Enhancement Imaging Increases Color Changes and Improves Visibility for Squamous Cell Carcinoma Suspicious Lesions in the Pharynx and Esophagus. Diagnostics, 11(11), 1971. https://doi.org/10.3390/diagnostics11111971






