Magnetic Resonance Spectroscopy for Risk Stratification of Sonographically Indeterminate Ovarian Neoplasms: Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Scan
2.3. MR Spectroscopy Acquisition
2.4. MRI Analysis
2.5. MR Spectroscopy Analysis
2.6. Pathological Diagnoses
2.7. Statistical Analysis
3. Results
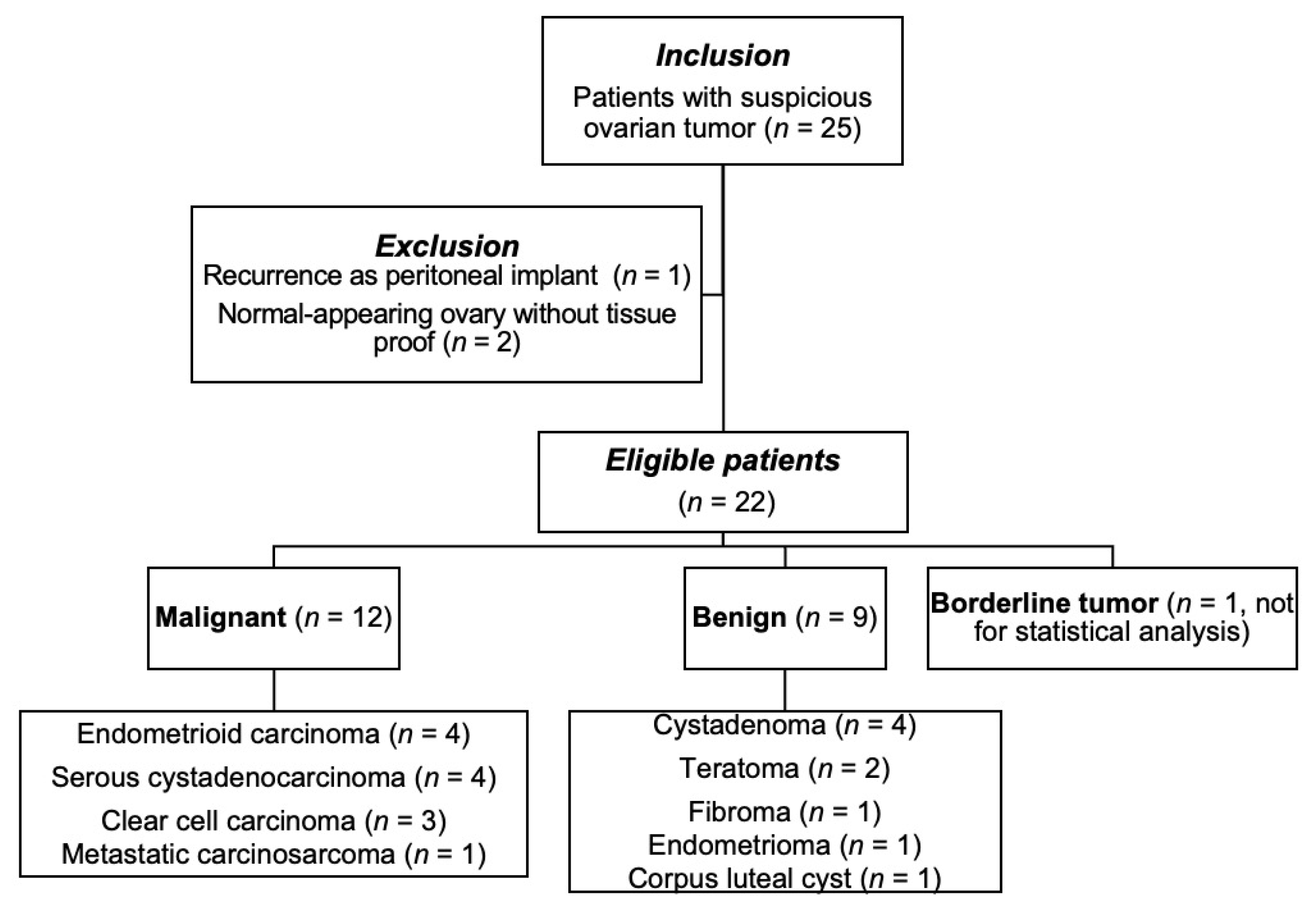
3.1. Patient Demographics and Pathological Diagnoses
3.2. Morphological MR Features (Modified O-RADS)
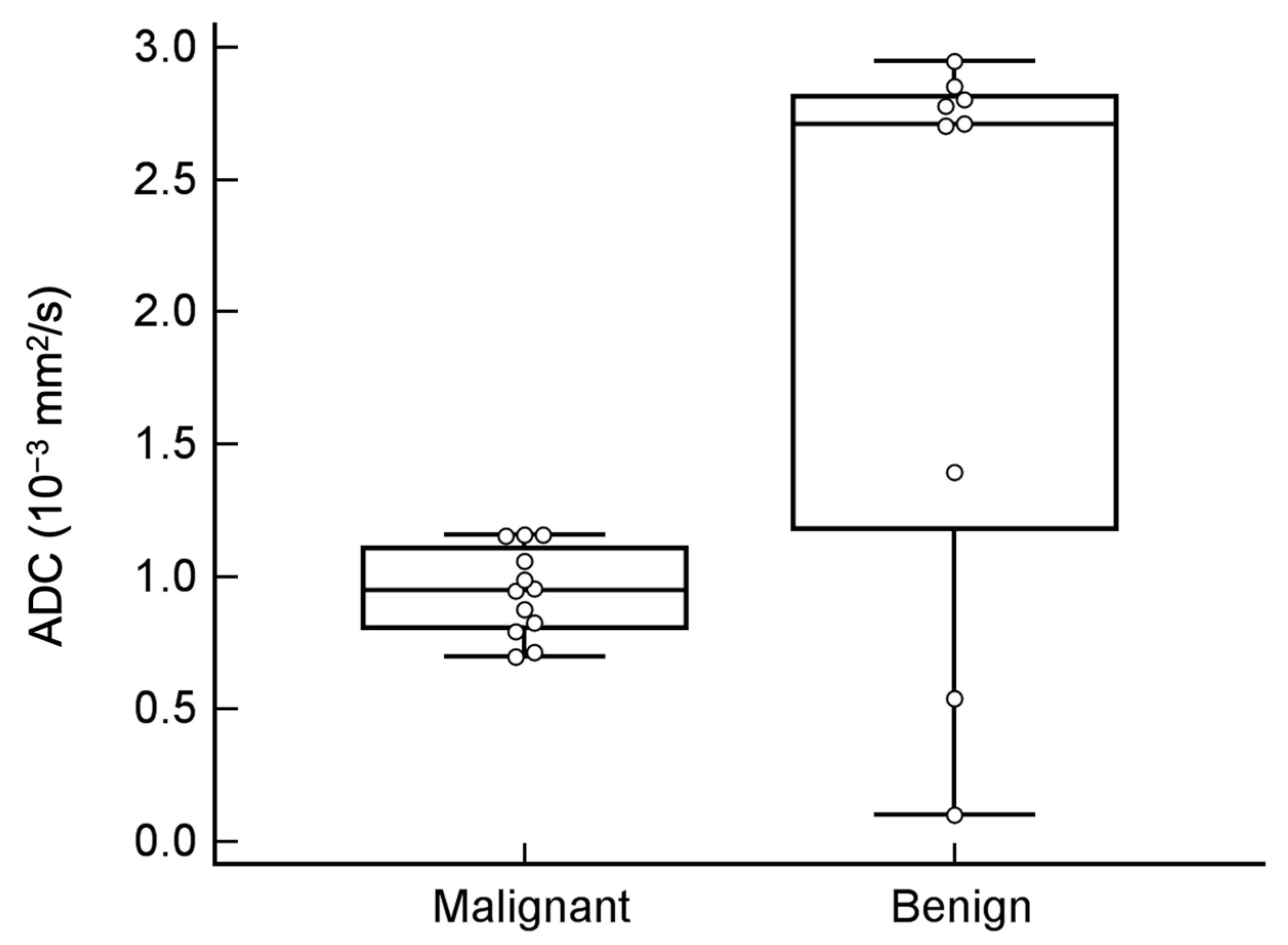
3.3. Value Addition of DWI and ADC Value in Tumor Classification
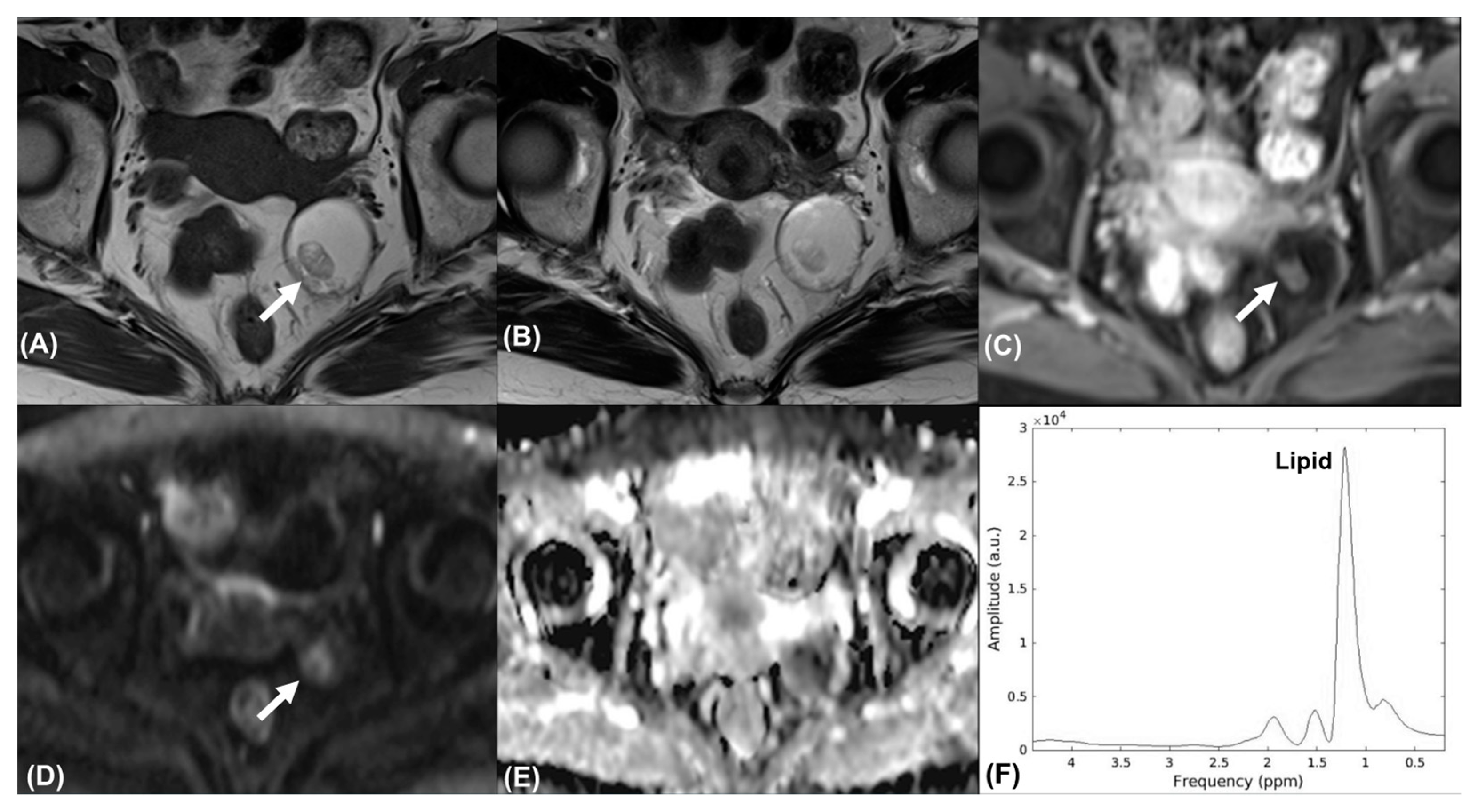
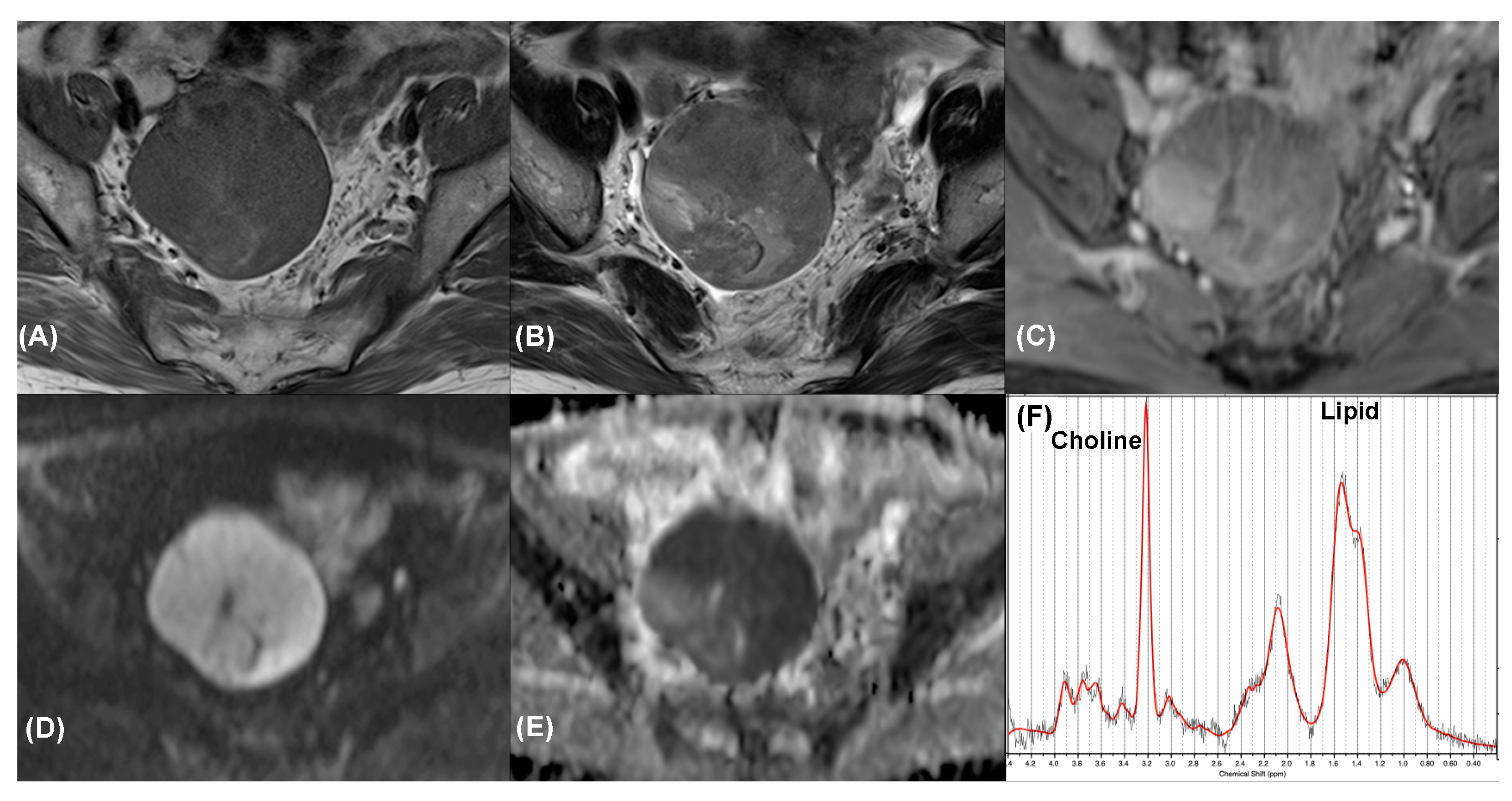
3.4. Additional Value of MRS
3.5. Borderline Tumor
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef]
- Andreotti, R.F.; Timmerman, D.; Strachowski, L.M.; Froyman, W.; Benacerraf, B.R.; Bennett, G.L.; Bourne, T.; Brown, D.L.; Coleman, B.G.; Frates, M.C.; et al. O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology 2020, 294, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Thomassin-Naggara, I.; Toussaint, I.; Perrot, N.; Rouzier, R.; Cuenod, C.A.; Bazot, M.; Darai, E. Characterization of complex adnexal masses: Value of adding perfusion- and diffusion-weighted MR imaging to conventional MR imaging. Radiology 2011, 258, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Thomassin-Naggara, I.; Aubert, E.; Rockall, A.; Jalaguier-Coudray, A.; Rouzier, R.; Darai, E.; Bazot, M. Adnexal masses: Development and preliminary validation of an MR imaging scoring system. Radiology 2013, 267, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, E.A.; Robbins, J.B.; Rockall, A.G.; Thomassin-Naggara, I. A systematic approach to adnexal masses discovered on ultrasound: The ADNEx MR scoring system. Abdom. Radiol. 2018, 43, 679–695. [Google Scholar] [CrossRef]
- Thomassin-Naggara, I.; Poncelet, E.; Jalaguier-Coudray, A.; Guerra, A.; Fournier, L.S.; Stojanovic, S.; Millet, I.; Bharwani, N.; Juhan, V.; Cunha, T.M.; et al. Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS MRI) Score for Risk Stratification of Sonographically Indeterminate Adnexal Masses. JAMA Netw. Open 2020, 3, e1919896. [Google Scholar] [CrossRef]
- ESUR Guidelines on Contrast Agents. Available online: https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf (accessed on 1 October 2021).
- Mansour, S.M.; Gomma, M.M.M.; Shafik, P.N. Proton MR spectroscopy and the detection of malignancy in ovarian masses. Br. J. Radiol. 2019, 92, 20190134. [Google Scholar] [CrossRef]
- De Souza, N.M.; Rockall, A.; Freeman, S. Functional MR Imaging in Gynecologic Cancer. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 205–222. [Google Scholar] [CrossRef]
- Ma, F.H.; Qiang, J.W.; Cai, S.Q.; Zhao, S.H.; Zhang, G.F.; Rao, Y.M. MR Spectroscopy for Differentiating Benign From Malignant Solid Adnexal Tumors. AJR Am. J. Roentgenol. 2015, 204, W724–W730. [Google Scholar] [CrossRef]
- Kang, Y.H.; Kim, M.Y.; Kim, K.T.; Kim, Y.J.; Suh, C.H.; Kim, J.M.; Hwang, S.O.; Park, S.; Cho, J.Y. H1Magnetic Resonance Spectroscopy of Cystic Ovarian Lesions. J. Korean Soc. Magn. Reson. Med. 2013, 17, 326–333. [Google Scholar] [CrossRef][Green Version]
- Lin, G.; Lin, Y.C.; Wu, R.C.; Yang, L.Y.; Lu, H.Y.; Tsai, S.Y.; Huang, Y.T.; Huang, Y.L.; Lu, K.Y.; Ng, K.K.; et al. Developing and validating a multivariable prediction model to improve the diagnostic accuracy in determination of cervical versus endometrial origin of uterine adenocarcinomas: A prospective MR study combining diffusion-weighted imaging and spectroscopy. J. Magn. Reson. Imaging 2018, 47, 1654–1666. [Google Scholar] [CrossRef]
- Reinhold, C.; Rockall, A.; Sadowski, E.A.; Siegelman, E.S.; Maturen, K.E.; Vargas, H.A.; Forstner, R.; Glanc, P.; Andreotti, R.F.; Thomassin-Naggara, I. Ovarian-Adnexal Reporting Lexicon for MRI: A White Paper of the ACR Ovarian-Adnexal Reporting and Data Systems MRI Committee. J. Am. Coll. Radiol. 2021, 18, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.; Devenyi, G.A.; Jezzard, P.; Hennessy, T.J.; Near, J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn. Reson. Med. 2017, 77, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, K.; Yamaguchi, K.; Nakazono, T.; Mizuguchi, M.; Aishima, S.; Yokoyama, M.; Irie, H. External validation of ADNEX MR SCORING system: A single-centre retrospective study. Clin. Radiol. 2019, 74, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.N.; Sarian, L.O.; Yoshida, A.; Araujo, K.G.; Barros, R.H.O.; Baiao, A.C.; Parente, D.B.; Derchain, S. Accuracy of the ADNEX MR scoring system based on a simplified MRI protocol for the assessment of adnexal masses. Diagn. Interv. Radiol. 2018, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Attina, G.; Spadola, S.; Caltabiano, R.; Farina, R.; Palmucci, S.; Zarbo, G.; Zarbo, R.; D’Arrigo, M.; Milone, P.; et al. MR imaging of ovarian masses: Classification and differential diagnosis. Insights Imaging 2016, 7, 21–41. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Meylaerts, L.J.; Laury, A.R.; Mortele, K.J. MRI features of ovarian fibroma and fibrothecoma with histopathologic correlation. AJR Am. J. Roentgenol. 2012, 198, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Kakite, S.; Nishihara, K.; Kanasaki, Y.; Harada, T.; Kigawa, J.; Kaminou, T.; Ogawa, T. Diagnostic accuracy of diffusion-weighted imaging in differentiating benign from malignant ovarian lesions. J. Magn. Reson. Imaging JMRI 2008, 28, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.Y.; Shin, Y.R.; Park, C.S.; Kim, K. The Value of Diffusion-Weighted Imaging in the Differential Diagnosis of Ovarian Lesions: A Meta-Analysis. PLoS ONE 2016, 11, e0149465. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuzaki, K.; Nishitani, H. Diffusion-weighted magnetic resonance imaging of ovarian tumors: Differentiation of benign and malignant solid components of ovarian masses. J. Comput. Assist. Tomogr. 2010, 34, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, L.; Horta, M.; Salvador, J.C.; Cunha, T.M. Benign ovarian lesions with restricted diffusion. Radiol. Bras. 2019, 52, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Jayasankaran, S.C.; Chelakkot, P.G.; Prabhakaran, P.; Sekhar, R. Role of Diffusion-Weighted Magnetic Resonance Imaging in the Evaluation of Ovarian Tumours. Indian J. Gynecol. Oncol. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Hagras, A.M.; Elhawary, T.M.; Hamisa, M. Diagnostic accuracy of diffusion weighted imaging & dynamic contrast enhanced MRI in differentiating benign & malignant ovarian masses for optimum possible surgical strategies. Fertil. Steril. 2018, 110, e132–e133. [Google Scholar] [CrossRef]
- Esseridou, A.; Di Leo, G.; Sconfienza, L.M.; Caldiera, V.; Raspagliesi, F.; Grijuela, B.; Hanozet, F.; Podo, F.; Sardanelli, F. In vivo detection of choline in ovarian tumors using 3D magnetic resonance spectroscopy. Investig. Radiol. 2011, 46, 377–382. [Google Scholar] [CrossRef]
- El Sorogy, L.; El Gaber, N.A.; Omran, E.; Elshamy, M.; Youssef, H. Role of diffusion MRI and proton magnetic resonance spectroscopy in characterization of ovarian neoplasms. Egypt. J. Radiol. Nucl. Med. 2012, 43, 99–106. [Google Scholar] [CrossRef]
- Stanwell, P.; Russell, P.; Carter, J.; Pather, S.; Heintze, S.; Mountford, C. Evaluation of ovarian tumors by proton magnetic resonance spectroscopy at three Tesla. Investig. Radiol. 2008, 43, 745–751. [Google Scholar] [CrossRef]
- Malek, M.; Pourashraf, M.; Gilani, M.M.; Gity, M. Improved Diagnostic Accuracy in Characterization of Adnexal Masses by Detection of Choline Peak Using 1H MR Spectroscopy in Comparison to Internal Reference at 3 Tesla. Asian Pac. J. Cancer Prev. 2015, 16, 5085–5088. [Google Scholar] [CrossRef][Green Version]
- Casarin, J.; Laganà, A.S.; Uccella, S.; Cromi, A.; Pinelli, C.; Gisone, B.; Borghi, C.; Cominotti, S.; Garzon, S.; Morotti, M.; et al. Surgical treatment of large adnexal masses: A retrospective analysis of 330 consecutive cases. Minim. Invasive Ther. Allied Technol. 2020, 29, 366–374. [Google Scholar] [CrossRef]
- Ma, F.H.; Li, Y.A.; Liu, J.; Li, H.M.; Zhang, G.F.; Qiang, J.W. Role of proton MR spectroscopy in the differentiation of borderline from malignant epithelial ovarian tumors: A preliminary study. J. Magn. Reson. Imaging JMRI 2019, 49, 1684–1693. [Google Scholar] [CrossRef] [PubMed]

| Malignant | Benign | |||
|---|---|---|---|---|
| Total number | 12 | 9 | ||
| Age, median (y) | 48 (33–84) | 41 (17–67) | ||
| Tumor size, mean (cm) | 6.9 (3.2–22) | 5.9 (4.3–17.7) | ||
| Histopathology | Endometrioid carcinoma Clear cell carcinoma Serous cystadenocarcinoma Metastatic carcinosarcoma | 4 3 4 1 | Teratoma Cystadenoma Fibroma Endometrioma Corpus luteal cyst | 2 4 1 1 1 |
| Menopausal status | ||||
| Premenopausal | 6 (50%) | 6 (66.7%) | ||
| Postmenopausal | 6 (50%) | 3 (33.3%) | ||
| FIGO stage | IA | 6 | ||
| IC | 4 | |||
| III | 2 | |||
| Treatment | 9 (100%) | |||
| Surgery alone | 6 (50%) | |||
| Surgery with adjuvant treatment | 6 (50%) |
| Malignant (n = 12) | Benign (n = 9) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Size (cm) | 7.9 | ± | 5.2 | 7.4 | ± | 4.3 | 0.808 |
| Mean ADC (10−3 mm2/s) | 0.9 | ± | 0.2 | 2.1 | ± | 1.1 | 0.034 * |
| Bilaterality | 0.553 | ||||||
| No | 11 | 91.7% | 7 | 77.8% | |||
| Yes | 1 | 8.3% | 2 | 22.2% | |||
| Morphology | <0.001 * | ||||||
| No | 0 | 0.0% | 8 | 88.9% | |||
| Yes | 12 | 100.0% | 1 | 11.1% | |||
| External contour | <0.001 * | ||||||
| No | 0 | 0.0% | 9 | 100.0% | |||
| Yes | 12 | 100.0% | 0 | 0.0% | |||
| Internal wall | <0.001 * | ||||||
| No | 0 | 0.0% | 9 | 100.0% | |||
| Yes | 12 | 100.0% | 0 | 0.0% | |||
| Internal septa | <0.001 * | ||||||
| No | 2 | 16.7% | 9 | 100.0% | |||
| Yes | 10 | 83.3% | 0 | 0.0% | |||
| Solid par | <0.001 * | ||||||
| No | 0 | 0.0% | 7 | 77.8% | |||
| Yes | 12 | 100.0% | 2 | 22.2% | |||
| Enhancement of solid part | <0.001 * | ||||||
| No | 1 | 8.3% | 8 | 88.9% | |||
| Yes | 11 | 91.7% | 1 | 11.1% | |||
| T1W | 0.021 * | ||||||
| No | 0 | 0.0% | 4 | 44.4% | |||
| Yes | 12 | 100.0% | 5 | 55.6% | |||
| T2W | <0.001 * | ||||||
| No | 1 | 8.3% | 8 | 88.9% | |||
| Yes | 11 | 91.7% | 1 | 11.1% | |||
| DWI | 0.002 * | ||||||
| No | 0 | 0.0% | 6 | 66.7% | |||
| Yes | 12 | 100.0% | 3 | 33.3% | |||
| Extraovarian lesion | 0.367 | ||||||
| No | 6 | 50.0% | 7 | 77.8% | |||
| Yes | 6 | 50.0% | 2 | 22.2% | |||
| Case | Histopathology | Malignancy | O-RADS | DWI | MRS |
|---|---|---|---|---|---|
| 1 | Serous cystadenocarcinoma | Yes | 5 | TP | FN |
| 2 | Serous cystadenocarcinoma | Yes | 5 | TP | FN |
| 3 | Metastatic carcinosarcoma | Yes | 5 | TP | FN |
| 4 | Clear cell carcinoma | Yes | 5 | FN | TP * |
| 5 | Clear cell carcinoma | Yes | 4 | FN | TP * |
| 6 | Clear cell carcinoma | Yes | 4 | FN | TP * |
| 7 | Endometrioid carcinoma | Yes | 4 | FN | TP * |
| 8 | Endometrioid carcinoma | Yes | 4 | TP | FN |
| 9 | Endometrioid carcinoma | Yes | 4 | TP | TP |
| 10 | Endometrioid carcinoma | Yes | 4 | TP | TP |
| 11 | Serous cystadenocarcinoma | Yes | 4 | TP | TP |
| 12 | Serous cystadenocarcinoma | Yes | 4 | TP | FN |
| 13 | Serous cystadenoma | No | 3 | TN | TN |
| 14 | Mucinous cystadenoma | No | 3 | TN | TN |
| 15 | Mucinous cystadenoma | No | 3 | TN | TN |
| 16 | Serous cystadenoma | No | 3 | TN | TN |
| 17 | Teratoma | No | 3 | FP | TN |
| 18 | Teratoma | No | 2 | FP | TN |
| 19 | Endometrioma | No | 2 | TN | TN |
| 20 | Fibroma | No | 2 | TN | TN |
| 21 | Corpus luteal cyst | No | 1 | TN | TN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Hsieh, C.-Y.; Huang, Y.-L.; Chen, K.; Huang, Y.-T.; Wu, R.-C.; Huang, H.-J.; Chou, H.-H.; Chao, A.; Lai, C.-H.; et al. Magnetic Resonance Spectroscopy for Risk Stratification of Sonographically Indeterminate Ovarian Neoplasms: Preliminary Study. Diagnostics 2021, 11, 1847. https://doi.org/10.3390/diagnostics11101847
Lin Y, Hsieh C-Y, Huang Y-L, Chen K, Huang Y-T, Wu R-C, Huang H-J, Chou H-H, Chao A, Lai C-H, et al. Magnetic Resonance Spectroscopy for Risk Stratification of Sonographically Indeterminate Ovarian Neoplasms: Preliminary Study. Diagnostics. 2021; 11(10):1847. https://doi.org/10.3390/diagnostics11101847
Chicago/Turabian StyleLin, Yenpo, Ching-Yi Hsieh, Yen-Ling Huang, Kueian Chen, Yu-Ting Huang, Ren-Chin Wu, Huei-Jean Huang, Hung-Hsueh Chou, Angel Chao, Chyong-Huey Lai, and et al. 2021. "Magnetic Resonance Spectroscopy for Risk Stratification of Sonographically Indeterminate Ovarian Neoplasms: Preliminary Study" Diagnostics 11, no. 10: 1847. https://doi.org/10.3390/diagnostics11101847
APA StyleLin, Y., Hsieh, C.-Y., Huang, Y.-L., Chen, K., Huang, Y.-T., Wu, R.-C., Huang, H.-J., Chou, H.-H., Chao, A., Lai, C.-H., & Lin, G. (2021). Magnetic Resonance Spectroscopy for Risk Stratification of Sonographically Indeterminate Ovarian Neoplasms: Preliminary Study. Diagnostics, 11(10), 1847. https://doi.org/10.3390/diagnostics11101847








