The Comparison and Interpretation of Machine-Learning Models in Post-Stroke Functional Outcome Prediction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. The PAC-CVD Program
2.3. Ethics
2.4. Eight ML Methods
2.5. Training and Validation
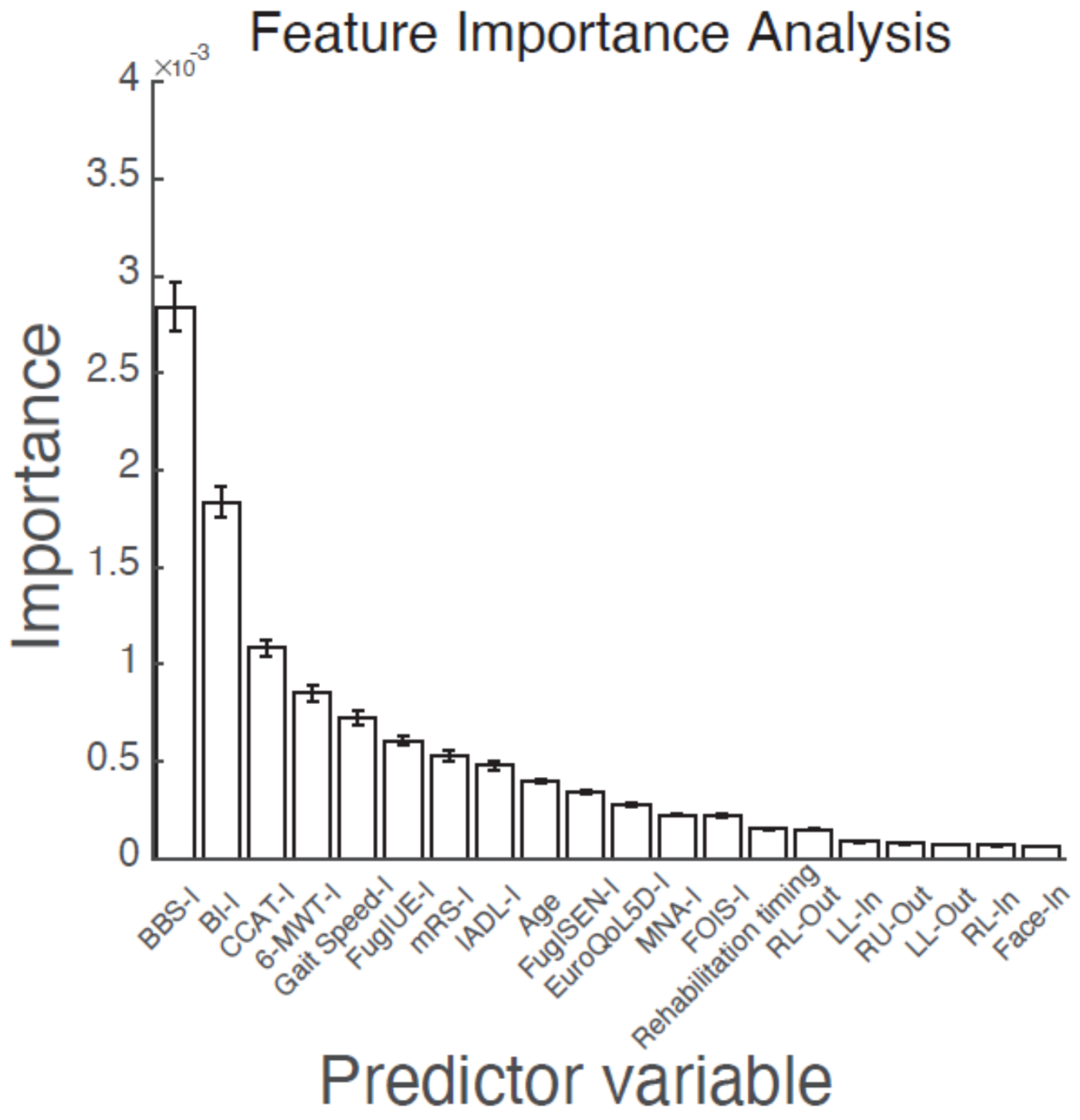
2.6. Feature Importance Analysis
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Performance of Post-Stroke Outcome Classification Models
3.3. Feature Importance for the Prognosis of BI at Discharge
3.4. Dependence and Heterogeneity of Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, C. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, A.W.; Roth, E.J.; Kristine, C.; Betts, H.B. Multivariate Analysis of Improvement and Outcome following Stroke Rehabilitation. Arch. Neurol. 1987, 44, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Kugler, C.; Altenhöner, T.; Lochner, P.; Ferbert, A. Does Age Influence Early Recovery from Ischemic Stroke? A Study from the Hessian Stroke Data Bank. J. Neurol. 2003, 250, 676–681. [Google Scholar] [CrossRef]
- Paker, N.; Buğdaycı, D.; Tekdöş, D.; Kaya, B.; Dere, C. Impact of Cognitive Impairment on Functional Outcome in Stroke. Stroke Res. Treat. 2010, 2010, 652612. [Google Scholar] [CrossRef] [Green Version]
- Mok, V.C.; Wong, A.; Lam, W.W.; Fan, Y.H.; Tang, W.K.; Kwok, T.; Hui, A.C.; Wong, K.S. Cognitive Impairment and Functional Outcome after Stroke Associated with Small Vessel Disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 560–566. [Google Scholar] [CrossRef]
- Nichols-Larsen, D.S.; Clark, P.C.; Zeringue, A.; Greenspan, A.; Blanton, S. Factors Influencing Stroke Survivors’ Quality of Life During Subacute Recovery. Stroke 2005, 36, 1480–1484. [Google Scholar] [CrossRef]
- Alexandrov, A.V.; Demchuk, A.M.; Felberg, R.A.; Christou, I.; Barber, P.A.; Burgin, W.S.; Malkoff, M.; Wojner, A.W.; Grotta, J.C. High Rate of Complete Recanalization and Dramatic Clinical Recovery During Tpa Infusion When Continuously Monitored with 2-Mhz Transcranial Doppler Monitoring. Stroke 2000, 31, 610–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, C.A.; Alexandrov, A.V.; Demchuk, A.M.; Saqqur, M.; Uchino, K.; Alvarez-Sabín, J. Improving the Predictive Accuracy of Recanalization on Stroke Outcome in Patients Treated with Tissue Plasminogen Activator. Stroke 2004, 35, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.P., Jr.; Davis, P.H.; Leira, E.C.; Chang, K.C.; Bendixen, B.H.; Clarke, W.R.; Woolson, R.F.; Hansen, M.D. Baseline Nih Stroke Scale Score Strongly Predicts Outcome after Stroke: A Report of the Trial of Org 10172 in Acute Stroke Treatment (Toast). Neurology 1999, 53, 126–131. [Google Scholar] [CrossRef]
- Paolucci, S.; Antonucci, G.; Grasso, M.G.; Bragoni, M.; Coiro, P.; de Angelis, D.; Fusco, F.R.; Morelli, D.; Venturiero, V.; Troisi, E.; et al. Functional Outcome of Ischemic and Hemorrhagic Stroke Patients after Inpatient Rehabilitation: A Matched Comparison. Stroke 2003, 34, 2861–2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, J.N.; Frampton, C.M.; Lyden, P.; Lees, K.R. Does Hemispheric Lateralization Influence Functional and Cardiovascular Outcomes after Stroke? An Analysis of Placebo-Treated Patients from Prospective Acute Stroke Trials. Stroke 2008, 39, 3335–3340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laredo, C.; Zhao, Y.; Rudilosso, S.; Renú, A.; Pariente, J.C.; Chamorro, Á.; Urra, X. Prognostic Significance of Infarct Size and Location: The Case of Insular Stroke. Sci. Rep. 2018, 8, 9498. [Google Scholar] [CrossRef]
- Schiemanck, S.K.; Kwakkel, G.; Post, M.W.; Prevo, A.J. Predictive Value of Ischemic Lesion Volume Assessed with Magnetic Resonance Imaging for Neurological Deficits and Functional Outcome Poststroke: A Critical Review of the Literature. Neurorehabil. Neural Repair 2006, 20, 492–502. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- King, D.; Wittenberg, R.; Patel, A.; Quayyum, Z.; Berdunov, V.; Knapp, M. The Future Incidence, Prevalence and Costs of Stroke in the Uk. Age Ageing 2020, 49, 277–282. [Google Scholar] [CrossRef]
- Mitchell, T.M. Machine Learning; McGraw-Hill Education: New York, NY, USA, 1997. [Google Scholar]
- Forkert, N.D.; Verleger, T.; Cheng, B.; Thomalla, G.; Hilgetag, C.C.; Fiehler, J. Multiclass Support Vector Machine-Based Lesion Mapping Predicts Functional Outcome in Ischemic Stroke Patients. PLoS ONE 2015, 10, e0129569. [Google Scholar] [CrossRef]
- Lin, W.Y.; Chen, C.H.; Tseng, Y.J.; Tsai, Y.T.; Chang, C.Y.; Wang, H.Y.; Chen, C.K. Predicting Post-Stroke Activities of Daily Living through a Machine Learning-Based Approach on Initiating Rehabilitation. Int. J. Med. Inform. 2018, 111, 159–164. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Imura, T.; Tanaka, R.; Imada, N.; Inagawa, T.; Araki, H.; Araki, O. Development and Validation of Machine Learning-Based Prediction for Dependence in the Activities of Daily Living after Stroke Inpatient Rehabilitation: A Decision-Tree Analysis. J. Stroke Cereb. Dis. 2020, 29, 105332. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.H. Stacked Generalization. Neural Netw. 1992, 5, 241–259. [Google Scholar] [CrossRef]
- Lai, C.L.; Tsai, M.M.; Luo, J.Y.; Liao, W.C.; Hsu, P.S.; Chen, H.Y. Post-Acute Care for Stroke—A Retrospective Cohort Study in Taiwan. Patient Prefer. Adherence 2017, 11, 1309–1315. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.Y.; Tsao, W.C.; Lin, R.T.; Chao, A.C. Three Years of the Nationwide Post-Acute Stroke Care Program in Taiwan. J. Chin. Med. Assoc. 2018, 81, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Sung, P.Y.; Liao, W.L.; Tsai, S.W. A Functional Recovery Profile for Patients with Stroke Following Post-Acute Rehabilitation Care in Taiwan. J. Chin. Med. Assoc. 2020, 119, 254–259. [Google Scholar] [CrossRef]
- Chong, D.K. Measurement of Instrumental Activities of Daily Living in Stroke. Stroke 1995, 26, 1119–1122. [Google Scholar] [CrossRef]
- Banks, J.L.; Marotta, C.A. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials: A Literature Review and Synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Richards, C.L.; Malouin, F.; Dumas, F.; Wood-Dauphinee, S. The Relationship of Gait Speed to Clinical Measures of Function and Muscle Activations During Recovery Post-Stroke. J. Biomech. 1993, 26, 324. [Google Scholar] [CrossRef]
- Eng, J.J.; Chu, K.S.; Dawson, A.S.; Kim, C.M.; Hepburn, K.E. Functional Walk Tests in Individuals with Stroke: Relation to Perceived Exertion and Myocardial Exertion. Stroke 2002, 33, 756–761. [Google Scholar] [CrossRef]
- Sullivan, K.J.; Tilson, J.K.; Cen, S.Y.; Rose, D.K.; Hershberg, J.; Correa, A.; Gallichio, J.; McLeod, M.; Moore, C.; Wu, S.S.; et al. Fugl-Meyer Assessment of Sensorimotor Function after Stroke: Standardized Training Procedure for Clinical Practice and Clinical Trials. Stroke 2011, 42, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial Psychometric Assessment of a Functional Oral Intake Scale for Dysphagia in Stroke Patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Kasner, S.E.; Chalela, J.A.; Luciano, J.M.; Cucchiara, B.L.; Raps, E.C.; McGarvey, M.L.; Conroy, M.B.; Localio, A.R. Reliability and Validity of Estimating the Nih Stroke Scale Score from Medical Records. Stroke 1999, 30, 1534–1537. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, Y.R.; Hong, J.P.; Chan, C.C.; Chang, L.C.; Shi, H.Y. Rehabilitative Post-Acute Care for Stroke Patients Delivered by Per-Diem Payment System in Different Hospitalization Paths: A Taiwan Pilot Study. Int. J. Qual. Health Care 2017, 29, 779–784. [Google Scholar] [CrossRef]
- Morone, G.; Paolucci, S.; Cherubini, A.; de Angelis, D.; Venturiero, V.; Coiro, P.; Iosa, M. Robot-Assisted Gait Training for Stroke Patients: Current State of the Art and Perspectives of Robotics. Neuropsychiatr. Dis. Treat. 2017, 13, 1303–1311. [Google Scholar] [CrossRef] [Green Version]
- Kwakkel, G.; Veerbeek, J.M.; van Wegen, E.E.; Wolf, S.L. Constraint-Induced Movement Therapy after Stroke. Lancet Neurol. 2015, 14, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Elsken, T.; Metzen, J.H.; Hutter, F. Neural Architecture Search: A Survey. J. Mach. Learn. Res. 2019, 20, 1997–2017. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, A.; Kapelner, A.; Bleich, J.; Pitkin, E. Peeking inside the Black Box: Visualizing Statistical Learning with Plots of Individual Conditional Expectation. J. Comput. Graph. Stat. 2015, 24, 44–65. [Google Scholar] [CrossRef]
- Kjersti, A.; Jullum, M.; Løland, A. Explaining Individual Predictions When Features Are Dependent: More Accurate Approximations to Shapley Values. arXiv 2019, 1903, 10464. [Google Scholar]
- Wee, J.Y.; Bagg, S.D.; Palepu, A. The Berg Balance Scale as a Predictor of Length of Stay and Discharge Destination in an Acute Stroke Rehabilitation Setting. Arch. Phys. Med. Rehabil. 1999, 80, 448–452. [Google Scholar] [CrossRef]
- Sandin, K.J.; Smith, B.S. The Measure of Balance in Sitting in Stroke Rehabilitation Prognosis. Stroke 1990, 21, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Wade, D.T.; Skilbeck, C.E.; Hewer, R.L. Predicting Barthel Adl Score at 6 Months after an Acute Stroke. Arch. Phys. Med. Rehabil. 1983, 64, 24–28. [Google Scholar] [PubMed]
- Peng, L.N.; Lu, W.H.; Liang, C.K.; Chou, M.Y.; Chung, C.P.; Tsai, S.L.; Chen, Z.J.; Hsiao, F.Y.; Chen, L.K. Functional Outcomes, Subsequent Healthcare Utilization, and Mortality of Stroke Postacute Care Patients in Taiwan: A Nationwide Propensity Score-Matched Study. J. Am. Med. Dir. Assoc. 2017, 18, 990.e7–990.e12. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, K.F.; Caro, J.J. The Barthel Index and Modified Rankin Scale as Prognostic Tools for Long-Term Outcomes after Stroke: A Qualitative Review of the Literature. Curr. Med. Res. Opin. 2007, 23, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Vanclay, F.; Cooper, B. Predicting Discharge Status at Commencement of Stroke Rehabilitation. Stroke 1989, 20, 766–769. [Google Scholar] [CrossRef] [Green Version]
- Ferro, J.M.; Mariano, G.; Madureira, S. Recovery from Aphasia and Neglect. Cerebrovasc. Dis. 1999, 9 (Suppl. 5), 6–22. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.B.; Lev, M.H.; Ay, H.; Singhal, A.B.; Greer, D.M.; Smith, W.S.; Harris, G.J.; Halpern, E.F.; Koroshetz, W.J.; Furie, K.L. The Prognosis for Aphasia in Stroke. J. Stroke Cerebrovasc. Dis. 2012, 21, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Laska, A.C.; Hellblom, A.; Murray, V.; Kahan, T.; von Arbin, M. Aphasia in Acute Stroke and Relation to Outcome. J. Intern. Med. 2001, 249, 413–422. [Google Scholar] [CrossRef]
- Carod-Artal, F.J.; Egido, J.A. Quality of Life after Stroke: The Importance of a Good Recovery. Cerebrovasc. Dis. 2009, 27 (Suppl. 1), 204–214. [Google Scholar] [CrossRef] [PubMed]
- Plowman, E.; Hentz, B.; Ellis, C., Jr. Post-Stroke Aphasia Prognosis: A Review of Patient-Related and Stroke-Related Factors. J. Eval. Clin. Pract. 2012, 18, 689–694. [Google Scholar] [CrossRef]
- Persky, R.W.; Turtzo, L.C.; McCullough, L.D. Stroke in Women: Disparities and Outcomes. Curr. Cardiol. Rep. 2010, 12, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargano, J.W.; Reeves, M.J. Sex Differences in Stroke Recovery and Stroke-Specific Quality of Life: Results from a Statewide Stroke Registry. Stroke 2007, 38, 2541–2548. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Tsuji, T.; Tsujiuchi, K.; Chino, N. Comorbidities in Stroke Patients as Assessed with a Newly Developed Comorbidity Scale. Am. J. Phys. Med. Rehabil. 1999, 78, 416–424. [Google Scholar] [CrossRef]
- Karatepe, A.G.; Gunaydin, R.; Kaya, T.; Turkmen, G. Comorbidity in Patients after Stroke: Impact on Functional Outcome. J. Rehabil. Med. 2008, 40, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Carsten, F.D.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar]
- Arboix, A.; Padilla, I.; Massons, J.; García-Eroles, L.; Comes, E.; Targa, C. Clinical Study of 222 Patients with Pure Motor Stroke. J. Neurol. Neurosurg. Psychiatry 2001, 71, 239–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arboix, A.; Alioc, J. Cardioembolic Stroke: Clinical Features, Specific Cardiac Disorders and Prognosis. Curr. Cardiol. Rev. 2010, 6, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, H.; Bernhardt, J.; Collier, J.M.; Sena, E.S.; McElduff, P.; Attia, J.; Pollack, M.; Howells, D.W.; Nilsson, M.; Calford, M.B. An Enriched Environment Improves Sensorimotor Function Post-Ischemic Stroke. Neurorehabilit. Neural Repair 2010, 24, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesh, A.; King-Shier, K.; Manns, B.J.; Hill, M.D.; Campbell, D.J.T. Money Is Brain: Financial Barriers and Consequences for Canadian Stroke Patients. Can. J. Neurol. Sci. 2017, 44, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.-C.; Chen, H.-F.; Peng, L.-N.; Lin, M.-H.; Chen, L.-K.; Liang, C.-K.; Lo, Y.-K.; Hwang, S.-J. Impact of Nutritional Status on Long-Term Functional Outcomes of Post-Acute Stroke Patients in Taiwan. Arch. Gerontol. Geriatr. 2011, 53, e149–e152. [Google Scholar] [CrossRef] [PubMed]


| Parameters | All | Group | ||
|---|---|---|---|---|
| Final BI > 60 | Final BI ≤ 60 | p Value | ||
| Patient number | 577 | 397 | 180 | |
| Age (year) | 64.6 ± 12.6 | 62.4 ± 12.3 | 69.4 ± 11.8 | <0.001 *** |
| Male/Female | 381/196 | 271 (71)/126 (64) | 110 (29)/70 (36) | 0.093 |
| Hemorrhagic stroke | 84 | 61 (73) | 23 (27) | 0.414 |
| Rehabilitation timing (day) | 13.2 ± 5.3 | 12.6 ± 5.1 | 14.5 ± 5.7 | <0.001 *** |
| BI-I | 48.3 ± 16.9 | 54.7 ± 14.6 | 34.4 ± 12.6 | <0.001 *** |
| BI-F | 71.2 ± 18.3 | 81.1 ± 10.5 | 49.3 ± 11.4 | <0.001 *** |
| ΔBI | 22.9 ± 14.8 | 26.4 ± 14.9 | 15.0 ± 10.9 | <0.001 *** |
| mRS-I | 3.5 ± 0.6 | 3.4 ± 0.6 | 3.9 ± 0.4 | <0.001 *** |
| FOIS-I | 5.7 ± 1.9 | 6 ± 1.6 | 5.0 ± 2.2 | <0.001 *** |
| MNA-I | 16.5 ± 5.3 | 16.6 ± 5.5 | 16.3 ± 4.7 | 0.263 |
| Euro-QoL-5D-I | 9.8 ± 1.6 | 9.5 ± 1.5 | 10.4 ± 1.6 | <0.001 *** |
| IADL-I | 1.7 ± 1.2 | 1.9 ± 1.2 | 1.1 ± 1.1 | <0.001 *** |
| BBS-I | 29.3 ± 17.4 | 36 ± 14.4 | 14.5 ± 13.8 | <0.001 *** |
| Gait speed-I (s) | 6.6 ± 9.6 | 7.8 ± 9.4 | 4.0 ± 9.4 | <0.001 *** |
| 6-MWT-I (m) | 115.1 ± 148.9 | 153.4 ± 157.0 | 30.6 ± 79.4 | <0.001 *** |
| FuglUE-I | 42.4 ± 20.0 | 47.2 ± 17.5 | 31.7 ± 20.8 | <0.001 *** |
| FuglSEN-I | 34.1 ±13.5 | 37.0 ± 10.7 | 27.9 ± 16.6 | <0.001 *** |
| CCAT-I | 10.7 ± 1.9 | 11.0 ± 1.49 | 9.9 ± 2.4 | <0.001 *** |
| Comorbidities | ||||
| Hypertension | 477 (77.5) | 305 (76.8) | 142 (78.9) | 0.583 |
| Diabetes mellitus | 220 (38.1) | 150 (37.8) | 70 (38.9) | 0.8 |
| Dyslipidemia | 271 (47.0) | 199 (50.1) | 72 (40.0) | 0.024 * |
| Atrial fibrillation | 54 (9.4) | 28 (7.1) | 26 (14.4) | 0.005 ** |
| Coronary arterial disease | 49 (8.5) | 31 (7.8) | 18 (10.0) | 0.382 |
| Chronic kidney disease | 25 (4.3) | 13 (3.3) | 12 (6.7) | 0.064 |
| Pulmonary disease | 17 (2.9) | 10 (2.5) | 7 (3.9) | 0.367 |
| Liver cirrhosis | 4 (0.7) | 2 (0.5) | 2 (1.1) | 0.415 |
| Hepatitis | 15 (2.6) | 14 (3.5) | 1 (0.6) | 0.038 * |
| Malignancy | 26 (4.5) | 13 (3.2) | 13 (7.2) | 0.034 * |
| Gout | 38 (6.6) | 33 (8.3) | 5 (2.8) | 0.013 * |
| Parkinsonism | 8 (1.4) | 4 (1.0) | 4 (2.2) | 0.248 |
| Dementia | 15 (2.6) | 8 (2.0) | 7 (3.9) | 0.19 |
| Old stroke | 124 (21.5) | 69 (17.4) | 55 (30.6) | <0.001 *** |
| Psychiatric disorder | 16 (2.8) | 13 (3.3) | 3 (1.7) | 0.276 |
| Complications | ||||
| Pneumonia | 29 (5.0) | 14 (3.5) | 15 (8.3) | 0.014 * |
| Urinary tract infection | 36 (6.2) | 20 (5.0) | 16 (8.9) | 0.076 |
| Stroke-in-evolution | 14 (2.4) | 7 (1.8) | 7 (3.9) | 0.124 |
| Gastrointestinal bleeding | 16 (2.8) | 10 (2.5) | 6 (3.3) | 0.581 |
| Cellulitis | 10 (1.7) | 7 (1.8) | 3 (1.7) | 0.934 |
| Model | AUC | ACC | Spe † | Sen † |
|---|---|---|---|---|
| Decision Tree (a) | 0.83 ± 0.048 | 0.817 ± 0.009 | 0.749 ± 0.067 | 0.828 ± 0.056 |
| Naïve Bayes (b) | 0.849 ± 0.008 | 0.786 ± 0.005 | 0.811 ± 0.074 | 0.744 ± 0.075 |
| kNN (c) | 0.856 ± 0.006 | 0.828 ± 0.006 | 0.709 ± 0.042 | 0.866 ± 0.041 |
| AdaBoost (d) | 0.871 ± 0.025 | 0.827 ± 0.011 | 0.792 ± 0.042 | 0.83 ± 0.04 |
| Linear Discriminant (e) | 0.876 ± 0.008 | 0.819 ± 0.009 | 0.785 ± 0.045 | 0.813 ± 0.046 |
| SVM (f) | 0.884 ± 0.003 | 0.831 ± 0.005 | 0.791 ± 0.022 | 0.841 ± 0.024 |
| Logistic Regression (g) | 0.886 ± 0.003 | 0.833 ± 0.005 | 0.794 ± 0.015 | 0.85 ± 0.041 |
| Stacking (h) | 0.886 ± 0.005 | 0.831 ± 0.005 | 0.859 ± 0.005 | 0.758 ± 0.011 |
| Random Forest (i) | 0.887 ± 0.003 | 0.829 ± 0.005 | 0.781 ± 0.036 | 0.829 ± 0.033 |
| p value | <0.001 *** a; b,c; d,e; f,g,h,i |
| Parameter | Final BI > 60 | Final BI ≤ 60 | p Value |
|---|---|---|---|
| BBS-I | 36.0 ± 14.4 | 14.5 ± 13.8 | <0.001 ** |
| BI-I | 54.7 ± 14.6 | 34.4 ± 12.6 | <0.001 ** |
| CCAT-I | 11.0 ± 1.49 | 9.9 ± 2.4 | <0.001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-C.; Chu, C.-L.; Chen, C.-K.; Chang, H.-N.; Wong, A.M.K.; Chen, Y.-P.; Pei, Y.-C. The Comparison and Interpretation of Machine-Learning Models in Post-Stroke Functional Outcome Prediction. Diagnostics 2021, 11, 1784. https://doi.org/10.3390/diagnostics11101784
Chang S-C, Chu C-L, Chen C-K, Chang H-N, Wong AMK, Chen Y-P, Pei Y-C. The Comparison and Interpretation of Machine-Learning Models in Post-Stroke Functional Outcome Prediction. Diagnostics. 2021; 11(10):1784. https://doi.org/10.3390/diagnostics11101784
Chicago/Turabian StyleChang, Shih-Chieh, Chan-Lin Chu, Chih-Kuang Chen, Hsiang-Ning Chang, Alice M. K. Wong, Yueh-Peng Chen, and Yu-Cheng Pei. 2021. "The Comparison and Interpretation of Machine-Learning Models in Post-Stroke Functional Outcome Prediction" Diagnostics 11, no. 10: 1784. https://doi.org/10.3390/diagnostics11101784
APA StyleChang, S.-C., Chu, C.-L., Chen, C.-K., Chang, H.-N., Wong, A. M. K., Chen, Y.-P., & Pei, Y.-C. (2021). The Comparison and Interpretation of Machine-Learning Models in Post-Stroke Functional Outcome Prediction. Diagnostics, 11(10), 1784. https://doi.org/10.3390/diagnostics11101784






