Accuracy of Ultrasonography and Magnetic Resonance Imaging for Preoperative Staging of Cervical Cancer—Analysis of Patients from the Prospective Study on Total Mesometrial Resection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
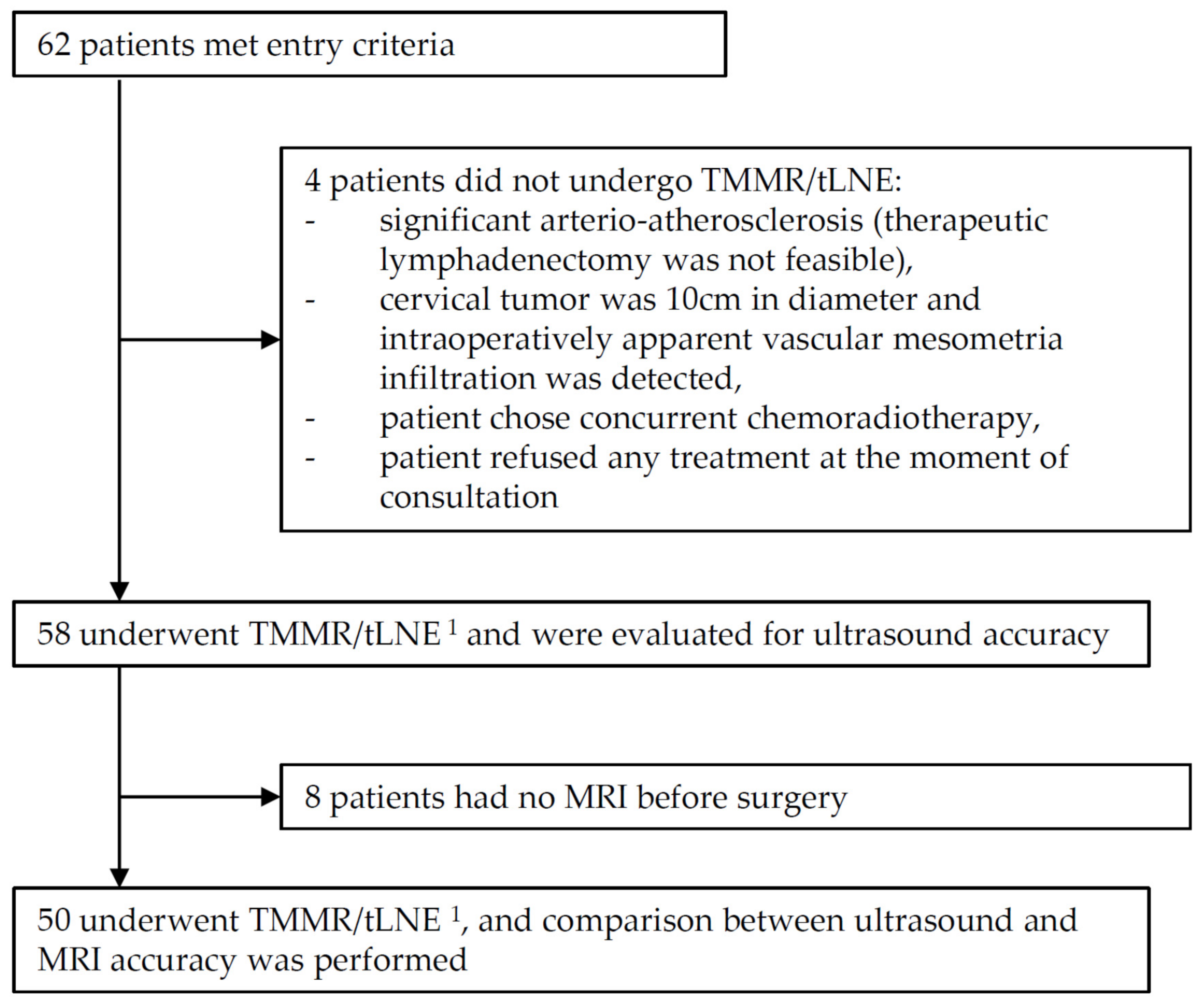
2.2. Participants
2.3. Index Tests
- Primary tumor detectable (yes/no).
- Primary tumor size (length, depth, width).
- Infiltration of parametria (yes/no).
- Infiltration of the uterine corpus (yes/no).
- Mesometrial (located in parametria) lymph node detection (yes/no).
- Infiltration of vagina wall/fornix (yes/no).
- Status of the anterior compartment (anterior to the cervix, border of the cervix and urinary bladder, and the urinary bladder).
- Status of the posterior compartment (posterior to the cervix, space between cervix and rectum, and the rectum).
- Suggested clinical stage (FIGO-2018, T from TNM (Tumor, Nodes, Metastases) system, and oT).
- Other (if noted; description).
2.3.1. Endovaginal/Transrectal Ultrasonography
2.3.2. Abdominal Ultrasonography for Regional Staging
2.3.3. MRI
2.3.4. Measurements and Stage Prediction
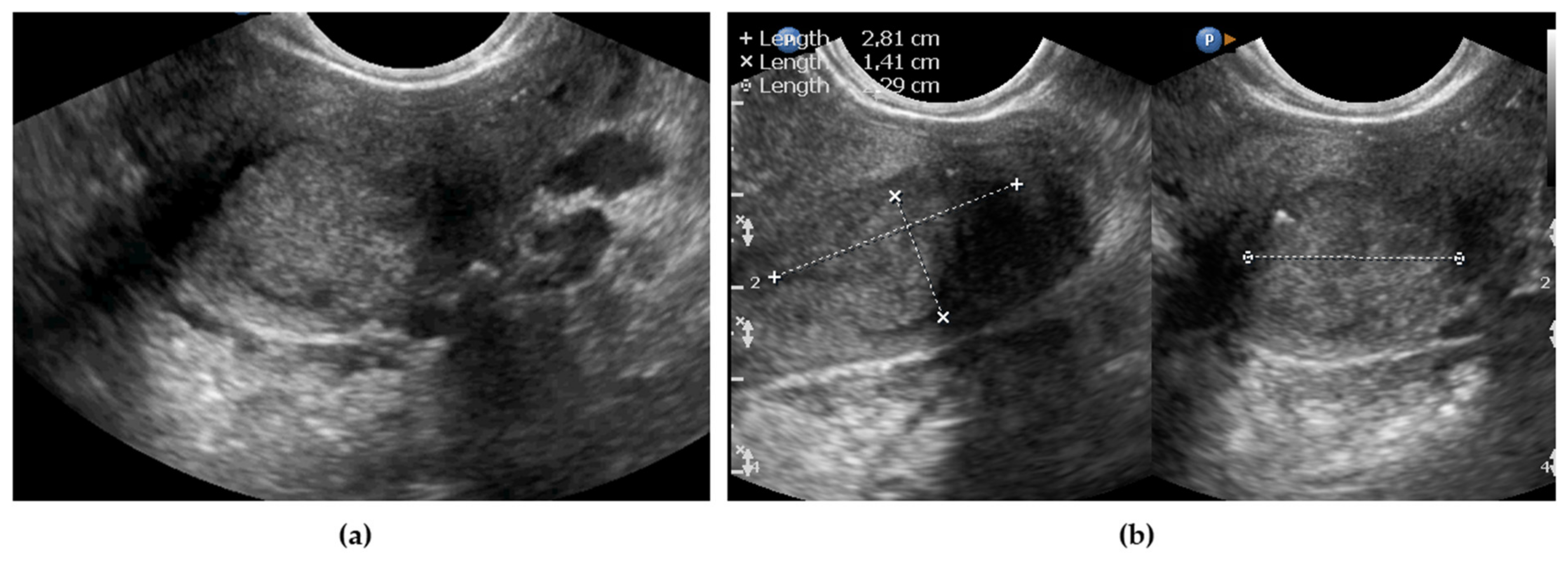
Measurements of the Primary Tumor
Stage Prediction
2.4. Reference Standard
2.5. Statistical Analysis
- sensitivity, true-positive rate (TPR):
- specificity, true-negative rate (TNR):
- precision, positive predictive value (PPV):
- negative predictive value (NPV):
- accuracy (ACC):
3. Results
3.1. Tumor Detection and Tumor Size
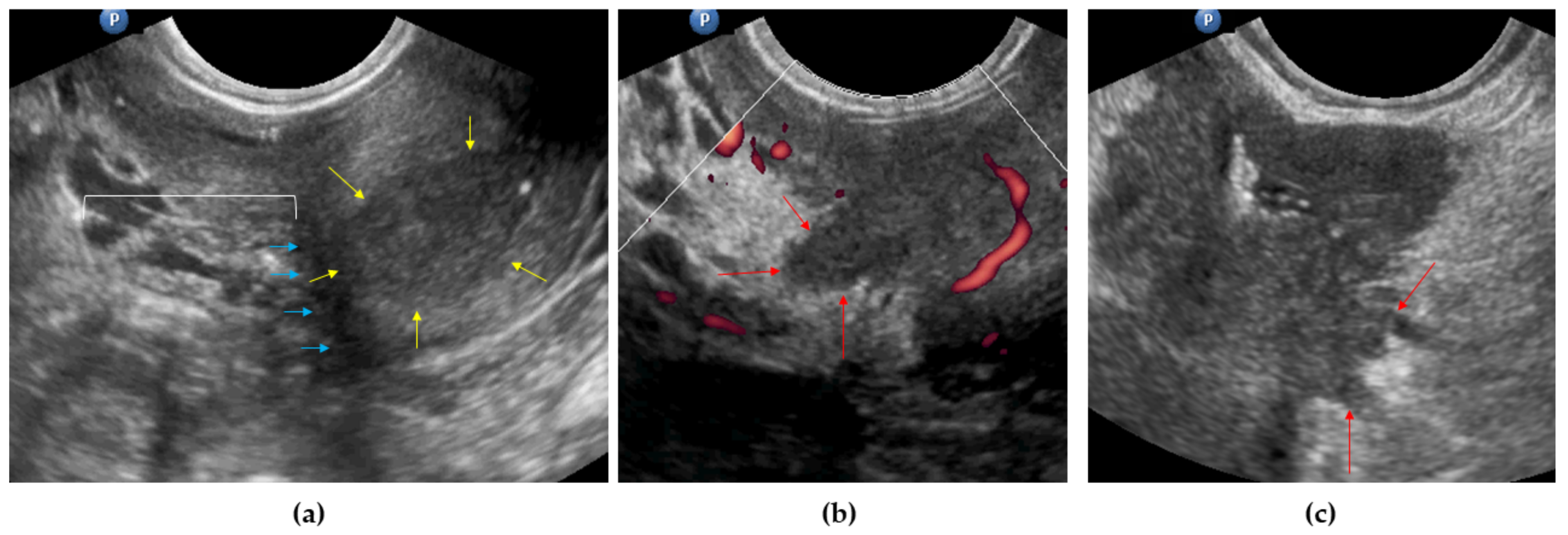
3.2. Parametria
3.3. Uterine Corpus Involvement
3.4. Mesometrial LNs
3.5. Vaginal Wall/Fornix Infiltration
3.6. Regions Anterior and Posterior to the Cervix
3.7. Stage Prediction
3.8. Regional LNs
4. Discussion
4.1. Tumor Detection and Tumor Size and Volume
4.2. Parametria
4.3. Uterine Corpus Involvement
4.4. Mesometrial LNs
4.5. Vaginal Wall/Fornix Infiltration
4.6. Regions Anterior and Posterior to the Cervix
4.7. Stage Prediction
4.8. Regional LNs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FIGO | Interational Federation of Gynecology and Obstetrics |
| LNs | lymph nodes |
| MRI | magnetic resonance imaging |
| oT | ontogenetic tumor (stage) |
| PET | position emision tomography |
| pT | pathological tumor (stage) |
| tLND | therapeutic lymph node dissection |
| TMMR | total mesometrial resection |
| TNM | Tumor Nodes Metastates (staging system) |
References
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European society of gynaecological oncology/european society for radiotherapy and oncology/European society of pathology guidelines for the management of patients with cervical cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Fischerová, D.; Cibula, D.; Stenhova, H.; Vondrichova, H.; Calda, P.; Zikan, M.; Freitag, P.; Slama, J.; Dundr, P.; Belacek, J. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cancer. Int. J. Gynecol. Cancer 2008, 18, 766–772. [Google Scholar] [CrossRef]
- Pinkavova, I.; Fischerova, D.; Zikan, M.; Burgetova, A.; Slama, J.; Svarovsky, J.; Dundr, P.; Dusek, L.; Cibula, D. Transrectal ultrasound and magnetic resonance imaging in the evaluation of tumor size following neoadjuvant chemotherapy for locally advanced cervical cancer. Ultrasound Obstet. Gynecol. 2013, 42, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Gaurilcikas, A.; Vaitkiene, D.; Cizauskas, A.; Inciura, A.; Svedas, E.; Maciuleviciene, R.; Di Legge, A.; Ferrandina, G.; Testa, A.C.; Valentin, L. Early-stage cervical cancer: Agreement between ultrasound and histopathological findings with regard to tumor size and extent of local disease. Ultrasound Obstet. Gynecol. 2011, 38, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, V.; Di Legge, A.; Valentini, A.L.; Gui, B.; Miccò, M.; Ludovisi, M.; Giansiracusa, C.; Testa, A.C.; Valentin, L. Agreement of two-dimensional and three-dimensional transvaginal ultrasound with magnetic resonance imaging in assessment of parametrial infiltration in cervical cancer. Ultrasound Obstet. Gynecol. 2015, 45, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Testa, A.; Gaurilcikas, A.; Di Legge, A.; Ameye, L.; Atstupenaite, V.; Valentini, A.L.; Gui, B.; Wallengren, N.-O.; Pudaric, S.; et al. Early-stage cervical cancer: Tumor delineation by magnetic resonance imaging and ultrasound—A European multicenter trial. Gynecol. Oncol. 2013, 128, 449–453. [Google Scholar] [CrossRef]
- Csutak, C.; Badea, R.; Bolboaca, S.; Ordeanu, C.; Nagy, V.M.; Fekete, Z.; Chiorean, L.; Dudea, S.M. Multimodal endocavitary ultrasound versus MRI and clinical findings in pre- and post-treatment advanced cervical cancer. Preliminary report. Med. Ultrason. 2016, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, D.; Li, H.; Cheng, G.; Wang, H. Application of transrectal ultrasound in guiding interstitial brachytherapy for advanced cervical cancer. J. Contemp. Brachyther. 2020, 12, 375–382. [Google Scholar] [CrossRef]
- Federico, M.; Hernandez-Socorro, C.R.; Ribeiro, I.; Martin, J.G.; Oramas, M.D.R.-B.; Saez-Bravo, M.L.; Jimenez, P.C.L. Prospective intra/inter-observer evaluation of pre-brachytherapy cervical cancer tumor width measured in TRUS and MR imaging. Radiat. Oncol. 2019, 14, 173. [Google Scholar] [CrossRef]
- Höckel, M.; Horn, L.C.; Hentschel, B.; Höckel, S.; Naumann, G. Total mesometrial resection: High resolution nerve-sparing radical hysterectomy based on developmentally defined surgical anatomy. Int. J. Gynecol. Cancer 2003, 13, 791–803. [Google Scholar] [CrossRef]
- Höckel, M.; Wolf, B.; Schmidt, K.; Mende, M.; Aktas, B.; Kimmig, R.; Dornhöfer, N.; Horn, L.-C. Surgical resection based on ontogenetic cancer field theory for cervical cancer: Mature results from a single-centre, prospective, observational, cohort study. Lancet Oncol. 2019, 20, 1316–1326. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Cormio, G.; Perego, P.; Milani, R.; Caruso, O.; Mangioni, C. Class II versus Class III radical hysterectomy in stage IB–IIA cervical cancer: A prospective randomized study. Gynecol. Oncol. 2001, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.A., III; Liu, P.Y.; Barrett, R.J., II; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- Rotman, M.; Sedlis, A.; Piedmonte, M.R.; Bundy, B.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int. J. Radiat. Oncol. 2006, 65, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Höckel, M.; Horn, L.-C.; Fritsch, H. Association between the mesenchymal compartment of uterovaginal organogenesis and local tumour spread in stage IB–IIB cervical carcinoma: A prospective study. Lancet Oncol. 2005, 6, 751–756. [Google Scholar] [CrossRef]
- Höckel, M.; Horn, L.-C.; Tetsch, E.; Einenkel, J. Pattern analysis of regional spread and therapeutic lymph node dissection in cervical cancer based on ontogenetic anatomy. Gynecol. Oncol. 2012, 125, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Education and Practical Standards Committee. Minimum training recommendations for the practice of medical ultrasound. Ultraschall. Med. 2006, 27, 79–105. [CrossRef]
- Timmerman, D.; Valentin, L.; Bourne, T.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the international ovarian tumor analysis (IOTA) group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.C.; Van Holsbeke, C.; Mascilini, F.; Timmerman, D. Dynamic and interactive gynecological ultrasound examination. Ultrasound Obstet. Gynecol. 2009, 34, 225–229. [Google Scholar] [CrossRef]
- Fischerova, D. Ultrasound scanning of the pelvis and abdomen for staging of gynecological tumors: A review. Ultrasound Obstet. Gynecol. 2011, 38, 246–266. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef]
- Höckel, M.; Hentschel, B.; Horn, L.-C. Association between developmental steps in the organogenesis of the uterine cervix and locoregional progression of cervical cancer: A prospective clinicopathological analysis. Lancet Oncol. 2014, 15, 445–456. [Google Scholar] [CrossRef]
- Kurman, R.J.; Amin, M.B. Protocol for the examination of specimens from patients with carcinomas of the cervix. Arch. Pathol. Lab. Med. 1999, 123, 55–61. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; De Vet, H.C.W.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef]
- Gebicki, J.; Szulczynski, B.; Kaminski, M. Determination of authenticity of brand perfume using electronic nose prototypes. Meas. Sci. Technol. 2015, 26, 125103. [Google Scholar] [CrossRef]
- Team, R. R Studio: Integrated Development for R; R Studio Inc.: Boston, MA, USA, 2015; Available online: http://www.rstudio.com (accessed on 17 June 2021).
- R-Project. A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 17 June 2021).
- Testa, A.C.; Ludovisi, M.; Manfredi, R.; Zannoni, G.; Gui, B.; Basso, D.; Di Legge, A.; Licameli, A.; Di Bidino, R.; Scambia, G.; et al. Transvaginal ultrasonography and magnetic resonance imaging for assessment of presence, size and extent of invasive cervical cancer. Ultrasound Obstet. Gynecol. 2009, 34, 335–344. [Google Scholar] [CrossRef]
- Alcazar, J.L.; Castillo, G.; Jurado, M.; Lopez-Garcia, G. Intratumoral blood flow in cervical cancer as assessed by transvaginal color doppler ultrasonography: Correlation with tumor characteristics. Int. J. Gynecol. Cancer 2003, 13, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Behn, U. The order of cancer: A theory of malignant progression by inverse morphogenesis. Front. Oncol. 2019, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Kubitschke, H.; Wolf, B.; Morawetz, E.; Horn, L.-C.; Aktas, B.; Behn, U.; Höckel, M.; Käs, J. Roadmap to local tumour growth: Insights from cervical cancer. Sci. Rep. 2019, 9, 12768. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Siegler, Y.; Segev, Y.; Mandel, R.; Siegler, E.; Auslander, R.; Lavie, O. The added benefit of transvaginal sonography in the clinical staging of cervical carcinoma. Acta Obstet. Gynecol. Scand. 2020, 99, 312–316. [Google Scholar] [CrossRef]
- Sodeikat, P.; Lia, M.; Martin, M.; Horn, L.-C.; Höckel, M.; Aktas, B.; Wolf, B. The importance of clinical examination under general anesthesia: Improving parametrial assessment in cervical cancer patients. Cancers 2021, 13, 2961. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, J.L.; García, E.; Machuca, M.; Quintana, R.; Escrig, J.; Chacón, E.; Mínguez, J.A.; Chiva, L. Magnetic resonance imaging and ultrasound for assessing parametrial infiltration in cervical cancer. A systematic review and meta-analysis. Med. Ultrason. 2020, 1, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Atun, R.; Ward, Z.; Scott, A.M.; Hricak, H.; Vargas, H.A. Diagnostic performance of conventional and advanced imaging modalities for assessing newly diagnosed cervical cancer: Systematic review and meta-analysis. Eur. Radiol. 2020, 30, 5560–5577. [Google Scholar] [CrossRef]
- Kimmig, R.; Aktas, B.; Buderath, P.; Rusch, P.; Heubner, M. Intraoperative navigation in robotically assisted compartmental surgery of uterine cancer by visualisation of embryologically derived lymphatic networks with indocyanine-green (ICG). J. Surg. Oncol. 2016, 113, 554–559. [Google Scholar] [CrossRef]
- Kimmig, R.; Rusch, P.; Buderath, P.; Aktas, B. Aortic utero-ovarian sentinel nodes and left infrarenal aortic lymph node dissection by ICG supported navigation. Gynecol. Oncol. Rep. 2017, 20, 22–23. [Google Scholar] [CrossRef]
- Kraima, A.; Derks, M.; Smit, N.; Van Munsteren, J.; Van der Velden, J.; Kenter, G.; DeRuiter, M. Lymphatic drainage pathways from the cervix uteri: Implications for radical hysterectomy? Gynecol. Oncol. 2014, 132, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Geppert, B.; Lönnerfors, C.; Bollino, M.; Arechvo, A.; Persson, J. A study on uterine lymphatic anatomy for standardization of pelvic sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2017, 145, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kimmig, R.; Buderath, P.; Rusch, P.; Aktas, B. Surgical anatomy of the ligamentous mesometrium and robotically assisted ICG-guided resection in cervical cancer. Gynecol. Oncol. Rep. 2017, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Kimmig, R.; Aktas, B.; Buderath, P.; Heubner, M. Robotically assisted peritoneal mesometrial resection (PMMR) in endometrial cancer supported by ICG labeling of the compartmental lymphatic system. Gynecol. Oncol. Rep. 2016, 16, 24. [Google Scholar] [CrossRef][Green Version]
- Buderath, P.; Rusch, P.; Mach, P.; Kimmig, R. Cancer field surgery in endometrial cancer: Peritoneal mesometrial resection and targeted compartmental lymphadenectomy for locoregional control. J. Gynecol. Oncol. 2021, 32, e7. [Google Scholar] [CrossRef]
- Horn, L.-C.; Hentschel, B.; Galle, D.; Bilek, K. Extracapsular extension of pelvic lymph node metastases is of prognostic value in carcinoma of the cervix uteri. Gynecol. Oncol. 2008, 108, 63–67. [Google Scholar] [CrossRef]
- Moloney, F.; Twomey, M.; Hewitt, M.; Barry, J.; Ryan, D.J. Comparison of MRI and high-resolution transvaginal sonography for the local staging of cervical cancer. J. Clin. Ultrasound 2016, 44, 78–84. [Google Scholar] [CrossRef]
- Arribas, S.; Alcazar, J.L.; Arraiza, M.; Benito, A.; Minguez, J.A.; Jurado, M. Three-dimensional transvaginal sonography and magnetic resonance imaging for local staging of cervical cancer: An agreement study. J. Ultrasound Med. 2016, 35, 867–873. [Google Scholar] [CrossRef]
- Wolf, B.; Ganzer, R.; Stolzenburg, J.-U.; Hentschel, B.; Horn, L.-C.; Höckel, M. Extended mesometrial resection (EMMR): Surgical approach to the treatment of locally advanced cervical cancer based on the theory of ontogenetic cancer fields. Gynecol. Oncol. 2017, 146, 292–298. [Google Scholar] [CrossRef]
- Pálsdóttir, K.; Fridsten, S.; Blomqvist, L.; Alagic, Z.; Fischerova, D.; Gaurilcikas, A.; Hasselrot, K.; Jäderling, F.; Testa, A.; Sundin, A.; et al. Inter-observer agreement of transvaginal ultrasound and magnetic resonance imaging in local staging of cervical cancer. Ultrasound Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Moro, F.; Gui, B.; Arciuolo, D.; Bertoldo, V.; Borzi, R.; Romeo, P.; Petta, F.; Cambi, F.; Pasciuto, T.; Zannoni, G.F.; et al. Fusion imaging of ultrasound and MRI in the assessment of locally advanced cervical cancer: A prospective study. Int. J. Gynecol. Cancer 2020, 30, 456–465. [Google Scholar] [CrossRef] [PubMed]
| Comparison Parameter | Histological Examination | ||
|---|---|---|---|
| Ultrasonography or MRI | Yes | No | |
| Yes | True positive (TP) | False positive (FP) | |
| No | False negative (FN) | True negative (TN) | |
| Clinical Parameters | Data |
|---|---|
| Age, years, median (range) | 48.5 (23–80) |
| BMI 1, kg/m2, median (IQR) | 26.6 (6.65) |
| Previous conization | 11 (19%) |
| Histological type | |
| Squamous cell carcinoma | 47 (81%) |
| Adenocarcinoma | 10 (17%) |
| Adenosquamous carcinoma | 1 (2%) |
| Histological grade | |
| G1 | 10 (17%) |
| G2 | 38 (66%) |
| G3 | 10 (17%) |
| LVSI 2 status | |
| Positive (+) | 17 (29%) |
| Negative (−) | 41 (71%) |
| Neoadjuvant chemotherapy | 0 |
| Therapeutic lymph node dissection | |
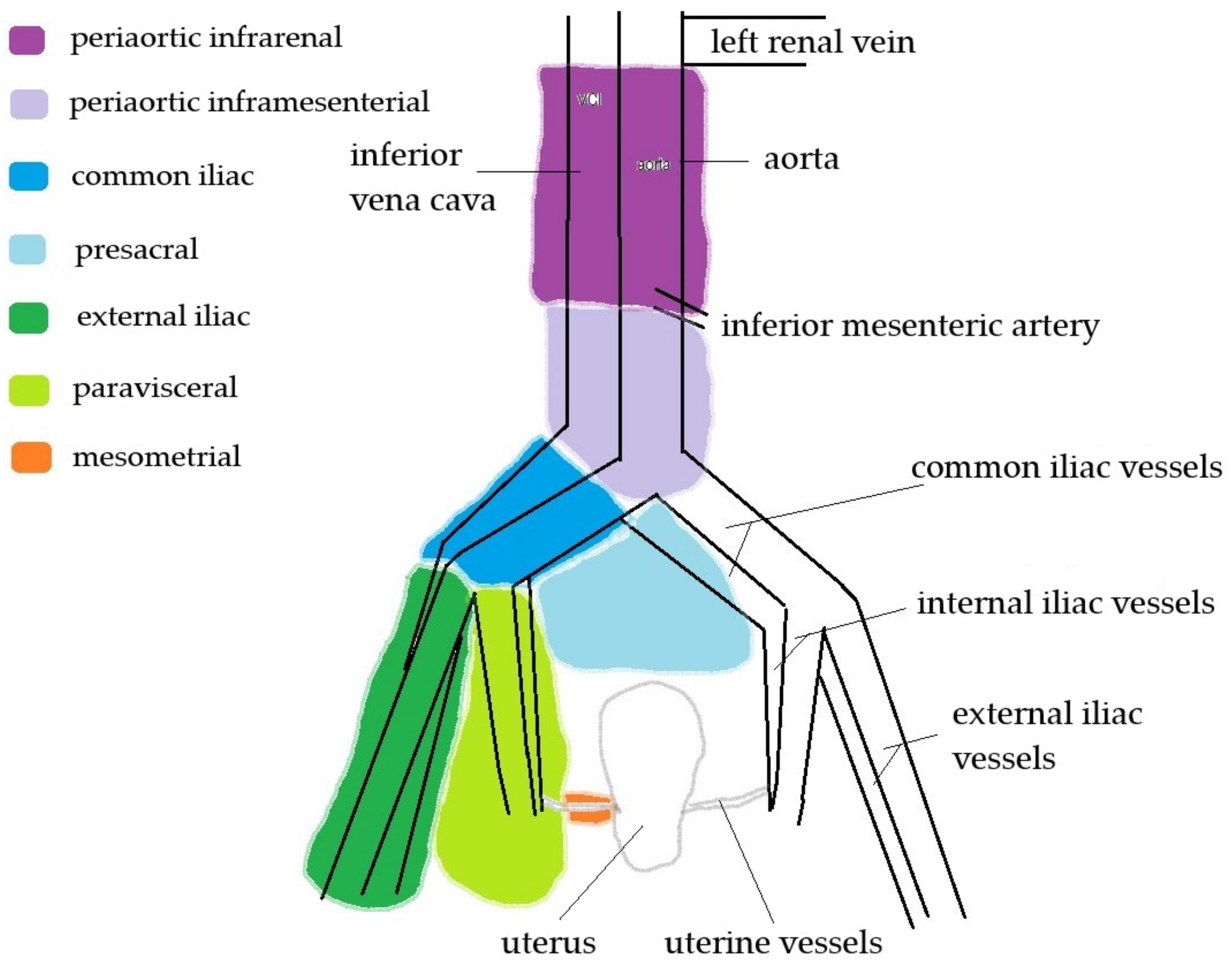
| External iliac, paravisceral, common iliac and presacral | 58 (100%) |
| Periaortic inframesenteric | 56 (97%) |
| Periaortic infrarenal | 9 (16%) |
| Number of LNs 3 removed per patient, median (range) | 57 (32–130) |
| Maximal tumor size, in histology 4, mm, median (IQR) | 32 (22.25) |
| pT stage, from TNM, 8th ed., 2016 | |
| pT1a | 1 (2%) |
| pT1b1 (< 4 cm) | 38 (65%) |
| pT1b2 (> 4 cm) | 14 (24%) |
| pT2a1 | 0 |
| pT2a2 | 1 (2%) |
| pT2b | 5 (8%) |
| pN stage, from TNM, 8th ed., 2016 | |
| pN0 | 38 (66%) |
| pN1 | 20 (34%) |
| Numer of metastatic LNs (including mesometrial) per patient, median (range) | 1 (1–7) |
| Pathological ontogenetic T stages | |
| (p)oT1 | 46 (79%) |
| (p)oT2 | 12 (21%) |
| Infiltrated extracervical tissues: | |
| Lateral parametria | 5 (9%) |
| Uterine corpus | 6 (10%) |
| Vaginal fornix/wall | 1 (2%) |
| Imaging Parameters | Ultrasound (n = 58) | Magnetic Resonance (n = 50) |
|---|---|---|
| Route | ||
| Transvaginal, n (%) | 24 (41%) | does not apply |
| Transrectal, n (%) | 34 (59%) | |
| Tumor (primary or residual post conization) detactable | ||
| Yes, n (%) | 49 (84%) | 41 (82%) |
| No, n (%) | 9 (16%) | 9 (18%) |
| Maximal tumor size, mm, median (IQR) | 25 (26.5) | 24 (28.5) |
| Lateral parametria involvement | 0 | 10 (20%) |
| Uterine corpus infiltration | 5 (9%) | 7 (14%) |
| Mesometrial LNs detectable | 1 (2%) | 0 |
| Vaginal wall/fornix infiltration | 1 (2%) | 13 (26%) |
| Anterior to the cervix involvement | 0 | 0 |
| Posterior to the cervix involvement | 0 | 2 (4%) |
| FIGO 2018 stage | ||
| IB1 | 19 (33%) | 21 (42%) |
| IB2 | 19 (33%) | 5 (10%) |
| IB3 | 19 (33%) | 4 (8%) |
| IIA1 | 0 | 3 (6%) |
| IIA2 | 1 (1%) | 6 (12%) |
| IIB | 0 | 9 (18%) |
| >IIB | 0 | 2 (4%) (IVA) |
| T stage, from TNM system, 8th ed., 2016 | ||
| 1b1 (< 4 cm) | 38 (66%) | 26 (52%) |
| 1b2 (> 4 cm) | 19 (33%) | 4 (8%) |
| 2a1 | 0 | 3 (6%) |
| 2a2 | 1 (1%) | 6 (12%) |
| 2b | 0 | 9 (18%) |
| >2b | 0 | 2 (4%) (4a) |
| Ontogentic T stage (preoperatively) | ||
| oT1 | 53 (91%) | 30 (60%) |
| oT2 | 5 (9%) | 18 (36%) |
| oT3 | 0 | 2 (4%) |
| Ultrasonography Assessment | Sensitivity, % | Specificity, % | PPV 1, % | NPV 2, % | Accuracy, % |
|---|---|---|---|---|---|
| Tumor detectable 3 | 94.12 (87.66–100.00) | 85.71 (59.79–100.00) | 97.96 (94.00–100.00) | 66.67 (35.87–97.46) | 93.10 (86.58–99.62) |
| Parametrial involvement | 0 (0.00–0.00) | 100 (100.00–100.00) | NC 4 | 89.66 (81.82–97.49) | 89.66 (81.82–97.49) |
| Uterine corpus involvement | 66.67 (28.95–100.00) | 98.08 (94.34–100.00) | 80.00 (44.94–100.00) | 96.23 (91.10–100.00) | 94.83 (89.13–100.00) |
| Vaginal fornix/wall involvement | 0 (0.00–0.00) | 98.21 (94.75–100.00) | 0 (0.00–0.00) | 96.49 (91.71–100.00) | 94.83 (89.13–100.00) |
| Imaging Assessment | Sensitivity, % | Specificity, % | PPV 1, % | NPV 2, % | Accuracy, % | p-Value 3 | |
|---|---|---|---|---|---|---|---|
| Tumor detectable 4 | Ultrasonography | 93.18 (85.73–100.00) | 83.33 (53.51–100.00) | 97.62 (93.00–100.00) | 62.50 (28.95–96.05) | 92.00 (84.48–99.52) | 0.56 |
| MRI | 90.91 (82.42–99.40) | 83.33 (53.51–100.00) | 97.56 (92.84–100.00) | 55.56 (23.09–88.02) | 90.00 (81.68–98.32) | ||
| Parametrial involvement | Ultrasonography | 0 (0.00–0.00) | 100 (100.00–100.00) | NC 5 | 92.00 (84.48–99.52) | 92.00 (84.48–99.52) | 0.32 |
| MRI | 25.00 (0.00–64.44) | 80.43 (68.97–91.90) | 10.00 (0.00–28.59) | 92.50 (84.34–100.00) | 76.00 (64.16–87.84) | ||
| Uterine corpus involvement | Ultrasonography | 66.67 (28.95–100.00) | 97.73 (93.32–100.00) | 80.00 (44.94–100.00) | 95.56 (89.53–100.00) | 94.00 (87.42–100.00) | 0.32 |
| MRI | 50.00 (9.99–90.00) | 90.91 (82.42–99.40) | 42.86 (6.20–79.52) | 93.02 (85.41–100.00) | 86.00 (76.3895.62) | ||
| Vaginal fornix/wallinvolvement | Ultrasonography | 0 (0.00–0.00) | 97.96 (94.00–100.00) | 0 (0.00–0.00) | 97.96 (94.00–100.00) | 96.00 (90.57–100.00) | 0.32 |
| MRI | 100 (100.00–100.00) | 75.51 (63.47–87.55) | 7.69 (0.00–22.18) | 100 (100.00–100.00) | 76.00 (64.16–87.84) | ||
| Tumor Size | Ultrasonography Mean Error | MRI Mean Error | Ultrasonography Better 1 n (%) | MRI Better 2 n (%) | Ultrasonography and MRI Equal 3 n (%) |
|---|---|---|---|---|---|
| By maximal size, mm | 5.70 | 8.84 | 20 (40%) | 22 (44%) | 8 (16%) |
| By mean size, mm | 4.6 | 5.9 | 21 (42%) | 20 (40%) | 9 (18%) |
| By volume 4, mm3 | 11775 | 15518 | 20 (40%) | 22 (44%) | 8 (16%) |
| By substitutive diameter 5 | 5.9 | 7.4 | 20 (40%) | 22 (44%) | 8 (16%) |
| Stage Classification | Ultrasound (N = 58) | MRI (N = 50) | p-Value 5 |
|---|---|---|---|
| FIGO 2018 2 | 68.97% (57.06–80.87%) | 42.00% (28.32–55.68%) | 0.0016 |
| T 3 | 79.31% (68.89–89.74%) | 52.00% (38.15–65.85%) | 0.0005 |
| oT 4 | 87.93% (79.55–96.31%) | 70.00% (57.30–82.70%) | 0.0045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stukan, M.; Buderath, P.; Szulczyński, B.; Gębicki, J.; Kimmig, R. Accuracy of Ultrasonography and Magnetic Resonance Imaging for Preoperative Staging of Cervical Cancer—Analysis of Patients from the Prospective Study on Total Mesometrial Resection. Diagnostics 2021, 11, 1749. https://doi.org/10.3390/diagnostics11101749
Stukan M, Buderath P, Szulczyński B, Gębicki J, Kimmig R. Accuracy of Ultrasonography and Magnetic Resonance Imaging for Preoperative Staging of Cervical Cancer—Analysis of Patients from the Prospective Study on Total Mesometrial Resection. Diagnostics. 2021; 11(10):1749. https://doi.org/10.3390/diagnostics11101749
Chicago/Turabian StyleStukan, Maciej, Paul Buderath, Bartosz Szulczyński, Jacek Gębicki, and Rainer Kimmig. 2021. "Accuracy of Ultrasonography and Magnetic Resonance Imaging for Preoperative Staging of Cervical Cancer—Analysis of Patients from the Prospective Study on Total Mesometrial Resection" Diagnostics 11, no. 10: 1749. https://doi.org/10.3390/diagnostics11101749
APA StyleStukan, M., Buderath, P., Szulczyński, B., Gębicki, J., & Kimmig, R. (2021). Accuracy of Ultrasonography and Magnetic Resonance Imaging for Preoperative Staging of Cervical Cancer—Analysis of Patients from the Prospective Study on Total Mesometrial Resection. Diagnostics, 11(10), 1749. https://doi.org/10.3390/diagnostics11101749








