Bioelectrical Impedance Analysis Derived-Phase Angle as a Pragmatic Tool to Detect Protein Energy Wasting among Multi-Ethnic Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Recruitment
2.2. Sample Size Requirement and Sampling Method
2.3. Research Instrument
2.4. Sociodemographic Data and Clinical Data
2.5. Nutritional Status Assessments
2.5.1. Bioelectrical Impedance Analysis Measurement (Index Test)
2.5.2. Anthropometric Measurements
MAMA (male) = [(MAC (cm) − π × TSF (cm))2/4π] − 10
MAMA (female) = [(MAC (cm) − π × TSF (cm))2/4π] − 6.5
2.5.3. Biochemical Data
2.5.4. Dietary Intake Assessment
2.5.5. PEW Diagnosis according to the ISRNM Criteria (Reference Standard)
2.6. Statistical Analyses
3. Results
3.1. Patient Recruitment
3.2. Patients’ Characteristics
3.3. Comparison of PhA across Patients’ Characteristics
3.4. Correlations between PhA with PEW Criteria and Body Composition in HD Patients
3.5. Predictors of PhA in HD Patients
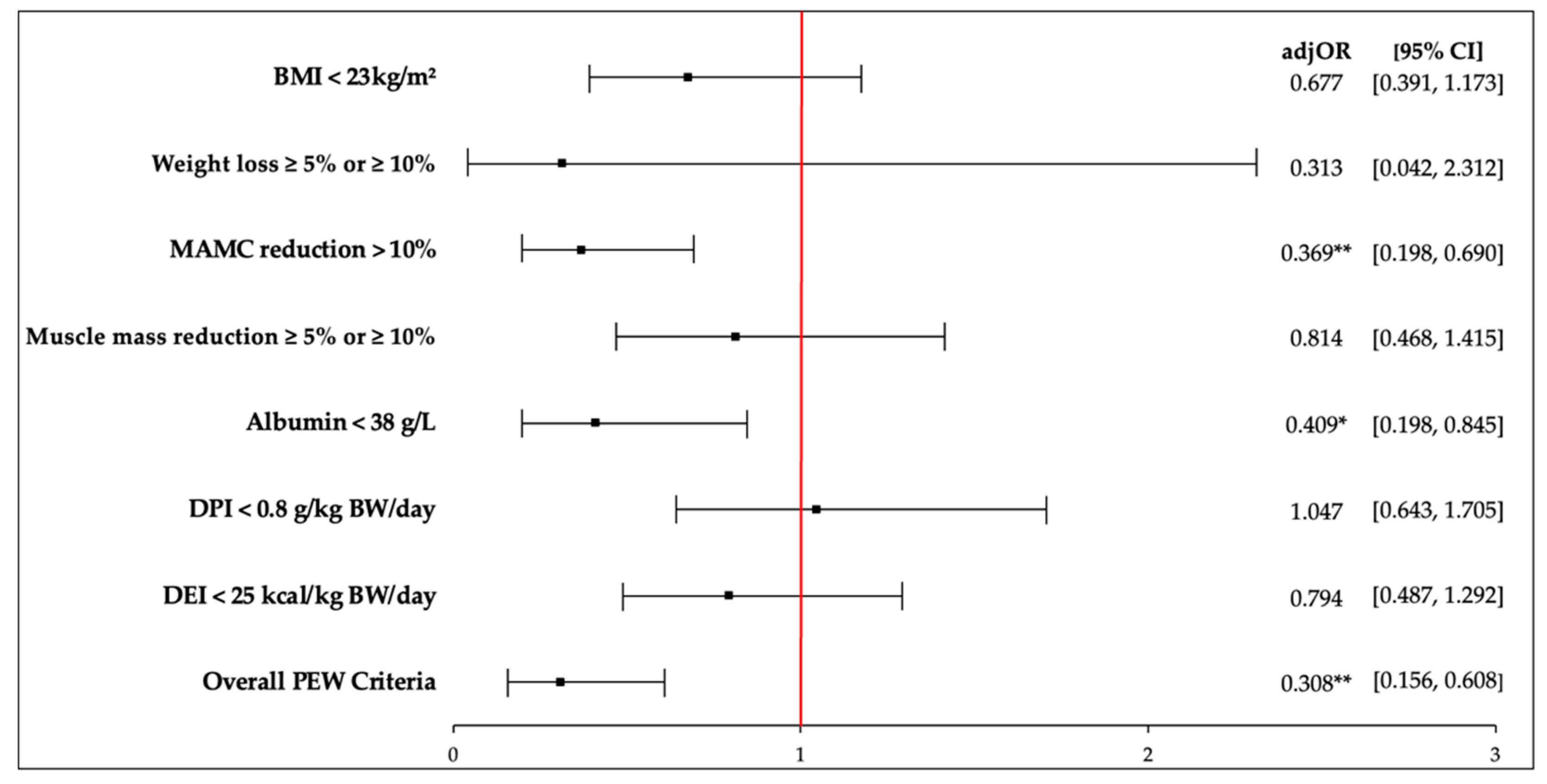
3.6. Associations of PhA and PEW Criteria in HD Patients
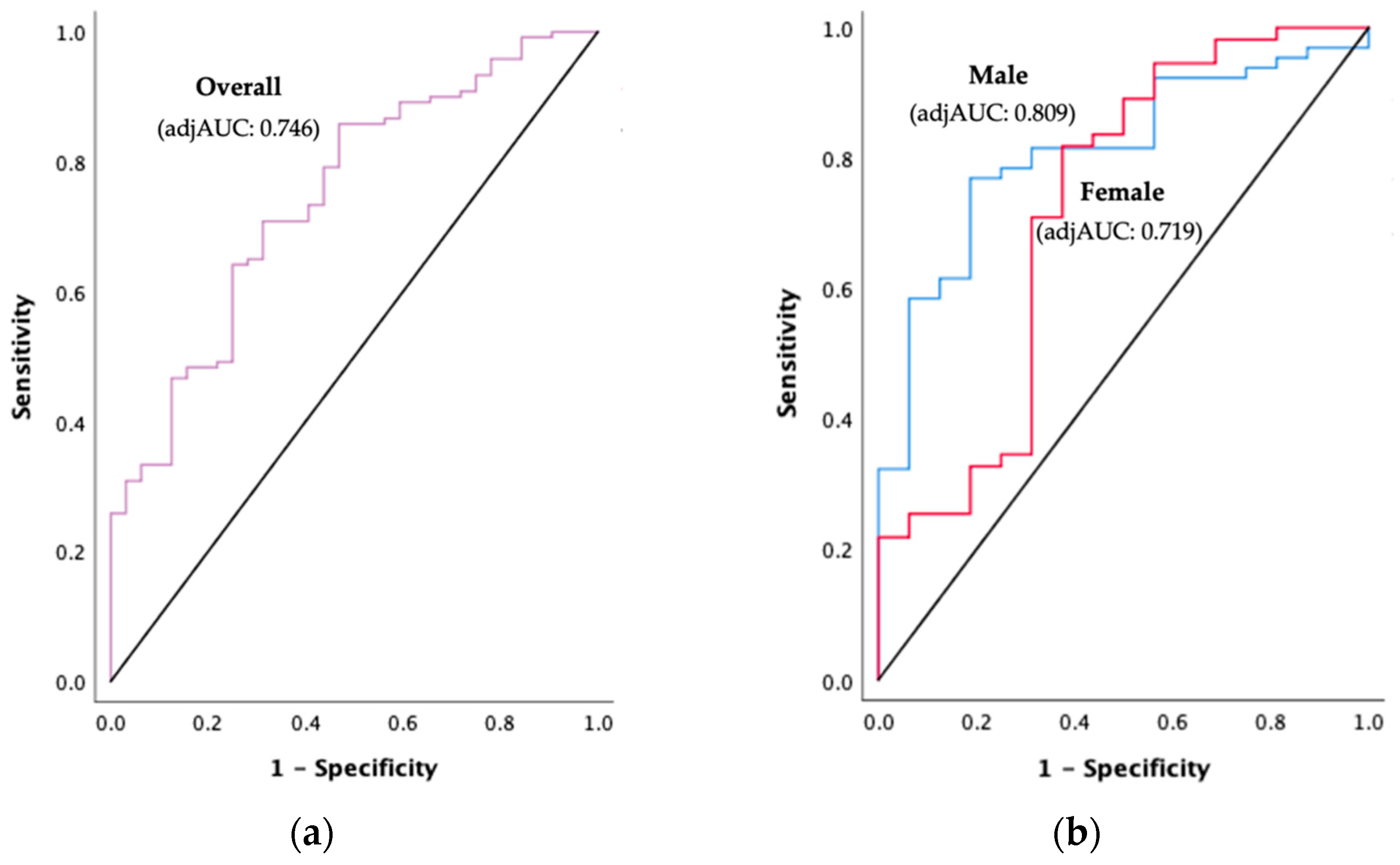
3.7. PhA Cut-Offs to Detect PEW in HD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccoli, G.B.; Moio, M.R.; Fois, A.; Sofronie, A.; Gendrot, L.; Cabiddu, G.; D’Alessandro, C.; Cupisti, A. The Diet and Haemodialysis Dyad: Three Eras, Four Open Questions and Four Paradoxes. A Narrative Review, Towards a Personalized, Patient-Centered Approach. Nutrients 2017, 9, 372. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Dai, L.; Mukai, H.; Lindholm, B.; Heimbürger, O.; Barany, P.; Stenvinkel, P.; Qureshi, A.R. Clinical global assessment of nutritional status as predictor of mortality in chronic kidney disease patients. PLoS ONE 2017, 12, e0186659. [Google Scholar] [CrossRef]
- Hyun, Y.Y.; Lee, K.B.; Han, S.H.; Kim, Y.; Kim, Y.H.; Lee, S.W.; Oh, Y.K.; Chae, D.W.; Ahn, C. Nutritional Status in Adults with Predialysis Chronic Kidney Disease: KNOW-CKD Study. J. Korean Med. Sci. 2017, 32, 257. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-Analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sarav, M.; Kovesdy, C.P. Protein Energy Wasting in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2018, 13, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Levin, A.; Lunney, M.; Osman, M.A.; Ye, F.; Ashuntantang, G.; Bellorin-Font, E.; Benghanem, G.M.; Ghnaimat, M.; Harden, P.; et al. Global Kidney Health Atlas: A Report by the International Society of Nephrology on the Global Burden of End-Stage Kidney Disease and Capacity for Kidney Replacement Therapy and Conservative Care across World Countries and Regions; International Society of Nephrology: Brussels, Belgium, 2019; p. 70. [Google Scholar]
- Chao, C.T.; Tang, C.H.; Cheng, R.W.Y.; Wang, M.Y.H.; Hung, K.Y. Protein-energy wasting significantly increases healthcare utilization and costs among patients with chronic kidney disease: A propensity-score matched cohort study. Curr. Med. Res. Opin. 2017, 33, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Leal Escobar, G.; Osuna Padilla, I.A.; Cano Escobar, B.; Moguel González, B.; Pérez Grovas, H.A.; Ruiz Ubaldo, S. Phase angle and mid arm circumference as predictors of protein energy wasting in renal replacement therapy patients. Nutr. Hosp. 2019, 36, 633–639. [Google Scholar]
- Ruperto, M.; Sánchez-Muniz, F.J.; Barril, G. Predictors of protein-energy wasting in haemodialysis patients: A cross-sectional study. J. Hum. Nutr. Diet. 2014, 29, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S.; Liang, D.H.; Liu, Y.; Zhong, X.S.; Zhang, D.S.; Ma, J. Bioelectrical Impedance Analysis–Derived Phase Angle Predicts Protein–Energy Wasting in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2019, 29, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Ogawa, M.; Kondo, H.; Suga, K.; Takahashi, T.; Itoh, H.; Tabata, Y. Bioelectrical impedance analysis-derived phase angle as a determinant of protein-energy wasting and frailty in maintenance hemodialysis patients: Retrospective cohort study. BMC Nephrol. 2020, 21, 438. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, W.; Pan, D.; Sun, G. Predicational ability of phase angle on protein energy wasting in kidney disease patients with renal replacement therapy: A cross-sectional study. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pupim, L.B.; Martin, C.J.; Ikizler, T.A. Assessment of Protein and Energy Nutritional Status. In Nutritional Management of Renal Disease, 3rd ed.; Kopple, J.D., Kalantar-Zadeh, K., Massry, S.G., Eds.; Academic Press: San Diego, CA, USA, 2013; p. 144. ISBN 978-0-12-391934-2. [Google Scholar]
- Your Body and You: A Guide to Phase Angle—InBody USA. Available online: https://inbodyusa.com/blogs/inbodyblog/your-body-and-you-a-guide-to-phase-angle/ (accessed on 29 June 2021).
- Barbosa-Silva, M.C.; Barros, A.J.D.; Wang, J.; Heymsfield, S.B.; Pierson, R.N., Jr. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ando, K.; Kobayashi, K.; Seki, T.; Hamada, T.; Machino, M.; Ota, K.; Morozumi, M.; Kanbara, S.; Ito, S.; et al. Low Bioelectrical Impedance Phase Angle Is a Significant Risk Factor for Frailty. BioMed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Zouridakis, A.; Simos, Y.V.; Verginadis, I.I.; Charalabopoulos, K.; Ragos, V.; Dounousi, E.; Boudouris, G.; Karkabounas, S.; Evangelou, A.; Peschos, D. Correlation of bioelectrical impedance analysis phase angle with changes in oxidative stress on end-stage renal disease patients, before, during, and after dialysis. Ren. Fail. 2016, 38, 738–743. [Google Scholar] [CrossRef]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer 2008, 8. [Google Scholar] [CrossRef]
- Fernandes, S.A.; de Mattos, A.A.; Tovo, C.V.; Marroni, C.A. Nutritional evaluation in cirrhosis: Emphasis on the phase angle. World J. Hepatol. 2016, 8, 1205. [Google Scholar] [CrossRef]
- Gerken, A.L.H.; Rohr-Kräutle, K.K.; Weiss, C.; Seyfried, S.; Reissfelder, C.; Vassilev, G.; Otto, M. Handgrip Strength and Phase Angle Predict Outcome After Bariatric Surgery. Obes. Surg. 2020, 31, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Moritoyo, T.; Kaufer-Horwitz, M.K.; Peine, S.; Norman, K.; Maisch, M.J.; Matsumoto, A.; Masui, Y.; Velázquez-González, A.; Domínguez-García, J.; et al. Ethnic differences in fat and muscle mass and their implication for interpretation of bioelectrical impedance vector analysis. Appl. Physiol. Nutr. Metab. 2019, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.; Egger, M.; Pocock, S.; Gøtzsche, P.; Vandenbroucke, J. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2007, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Lazarus, J.M.; Lew, N.L.; Ma, L.; Lowrie, E.G. Bioimpedance norms for the hemodialysis population. Kidney Int. 1997, 52, 1617–1621. [Google Scholar] [CrossRef][Green Version]
- Abad, S.; Sotomayor, G.; Vega, A.; Pérez de José, A.; Verdalles, U.; Jofré, R.; López-Gómez, J.M. The phase angle of the electrical impedance is a predictor of long-term survival in dialysis patients. Nefrologia 2011, 31, 670–676. [Google Scholar] [CrossRef]
- Leeflang, M.M.; Deeks, J.J.; Gatsonis, C.; Bossuyt, P.M.; Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Ann. Intern. Med. 2008, 149, 889–897. [Google Scholar] [CrossRef]
- BCM-Body Composition Monitor; Fresenius Medical Care: Hong Kong, 2021; pp 1–7. Available online: http://www.fmc-my.com/pdf/body_composition_monitor/Body%20Composition%20Monitor.pdf (accessed on 29 June 2021).
- Keane, D.F.; Baxter, P.; Lindley, E.; Moissl, U.; Pavitt, S.; Rhodes, L.; Wieskotten, S. The Body Composition Monitor: A Flexible Tool for Routine Fluid Management Across The Haemodialysis Population. Biomed. Phys. Eng. Express 2017, 3, 035017. [Google Scholar] [CrossRef]
- Abbas, S.R.; Zhu, F.; Kaysen, G.A.; Kotanko, P.; Levin, N.W. Effect Of Change In Fluid Distribution In Segments In Hemodialysis Patients At Different Ultrafiltration Rates On Accuracy Of Whole Body Bioimpedance Measurement. J. Appl. Physiol. 2014, 116, 1382–1389. [Google Scholar] [CrossRef]
- Chen, H.S.; Lee, K.C.; Cheng, C.T.; Hou, C.C.; Liou, H.H.; Lin, C.J.; Lim, P.S. Application of Bioimpedance Spectroscopy in Asian Dialysis Patients (ABISAD): Serial follow-up and dry weight evaluation. Clin. Kidney J. 2012, 6, 29–34. [Google Scholar] [CrossRef]
- Hou, G.C.; Gan, H.; Sun, X.; Li, J. Use of body composition measurements to guide the assessment of dry weight in anuric dialysis patients: Improvements in blood pressure control. Biochem. Biophys. Rep. 2019, 17, 191–196. [Google Scholar] [CrossRef]
- Kumar, S.; Dutt, A.; Hemraj, S.; Bhat, S.; Manipadybhima, B. Phase Angle Measurement in Healthy Human Subjects through Bio-Impedance Analysis. Iran J. Basic Med. Sci. 2012, 15, 1180–1184. [Google Scholar] [PubMed]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; de Ridder, H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Heymsfield, S.B.; McManus, C.; Smith, J.; Stevens, V.; Nixon, D.W. Anthropometric measurement of muscle mass: Revised equations for calculating bone-free arm muscle area. Am. J. Clin. Nutr. 1982, 36, 680–690. [Google Scholar] [CrossRef]
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI National Kidney Foundation. Am. J. Kidney Dis. 2000, 35, S1–S140. Available online: https://www.kidney.org/sites/default/files/docs/kdoqi2000nutritiongl.pdf (accessed on 18 September 2021).
- Levy, J.; Brown, E.; Lawrence, A. Oxford Handbook of Dialysis, 4th ed.; Oxford University Press: Oxford, UK, 2016; p. 166. [Google Scholar]
- Tee, E.S.; Mohd Ismail, N.; Mohd Nasir, A.; Khatijah, I. Nutrient Composition of Malaysian Foods, 4th ed.; Institute for Medical Research Malaysia: Kuala Lumpur, Malaysia, 1997; ISBN 978-967-99909-8-0. [Google Scholar]
- Singapore Health Promotion Board. Energy & Nutrient Composition of Food. Available online: https://focos.hpb.gov.sg/eservices/ENCF/ (accessed on 30 June 2021).
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Nutrition in CKD Guideline Work Group. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Black, A.E. Critical evaluation of energy intake using the goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Bross, R.; Shapiro, B.B.; Morrison, G.; Kopple, J.D. Dietary energy requirements in relatively healthy maintenance hemodialysis patients estimated from long-term metabolic studies. Am. J. Clin. Nutr. 2016, 103, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Waqas, K.; Tan, R.C.; Voortman, T.; Ikram, M.A.; Nijsten, T.E.C.; de Groot, L.C.P.G.M.; Uitterlinden, A.G.; Zillikens, M.C. The association between dietary and skin advanced glycation end products: The Rotterdam Study. Am. J. Clin. Nutr. 2020, 112, 129–137. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; p. 177. [Google Scholar]
- NRR|National Renal Registry: 24th Report of the Malaysian Dialysis & Transplant Registry 2016. Available online: https://msn.org.my/nrr/mdtr2016.jsp (accessed on 19 July 2021).
- Milanovic, Z.; Pantelić, S.; Trajković, N.; Sporis, G.; Kostić, R.; James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 2013, 8, 549. [Google Scholar] [CrossRef]
- Zs-Nagy, I. Aging of Cell Membranes: Facts and Theories. Aging 2014, 39, 62–85. [Google Scholar] [CrossRef]
- Kuchnia, A.J.; Teigen, L.M.; Cole, A.J.; Mulasi, U.; Gonzalez, M.C.; Heymsfield, S.B.; Vock, D.M.; Earthman, C.P. Phase Angle and Impedance Ratio: Reference Cut-Points From The United States National Health And Nutrition Examination Survey 1999–2004 From Bioimpedance Spectroscopy Data. JPEN J. Parenter. Enter. Nutr. 2016, 41, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Iguacel, C.; González-Parra, E.; Barril-Cuadrado, G.; Sánchez, R.; Egido, J.; Ortiz-Arduán, A.; Carrero, J.J. Defining protein-energy wasting syndrome in chronic kidney disease: Prevalence and clinical implications. Nefrologia 2014, 34, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Vegine, P.M.; Fernandes, A.C.; Torres, M.R.; Silva, M.I.; Avesani, C.M. Assessment of methods to identify protein-energy wasting in patients on hemodialysis. J. Bras. Nefrol. 2011, 33, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L. Regression Dilution Bias: Tools for Correction Methods and Sample Size Calculation. Upsala J. Med. Sci. 2012, 117, 279–283. [Google Scholar] [CrossRef]
- Bennett, D.A.; Landry, D.; Little, J.; Minelli, C. Systematic review of statistical approaches to quantify, or correct for, measurement error in a continuous exposure in nutritional epidemiology. BMC Med. Res. Methodol. 2017, 17, 146. [Google Scholar] [CrossRef]
- Wulan, S.N.; Westerterp, K.R.; Plasqui, G. Ethnic Differences in body composition and the associated metabolic profile: A comparative study between Asians and Caucasians. Maturitas 2010, 65, 315–319. [Google Scholar] [CrossRef]
- Hui, D.; Dev, R.; Pimental, L.; Park, M.; Cerana, M.A.; Liu, D.; Bruera, E. Association between Multi-Frequency Phase Angle And Survival In Patients With Advanced Cancer. J. Pain Symptom Manag. 2017, 53, 571–577. [Google Scholar] [CrossRef]
- Raimann, J.G.; Abbas, S.R.; Liu, L.; Zhu, F.; Larive, B.; Kotanko, P.; Levin, N.W.; Kaysen, G.A. Agreement of Single-and Multi-Frequency Bioimpedance Measurements in Hemodialysis Patients: An Ancillary Study of the Frequent Hemodialysis Network Daily Trial. Nephron Clin. Pract. 2014, 128, 115–126. [Google Scholar] [CrossRef]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. 2016, 26, 297–307. [Google Scholar] [CrossRef] [PubMed]
| Variables | n (%) | Median (q1–q3) | Range |
|---|---|---|---|
| Age (years) | 58.5 (50.0–65.8) | 25–77 | |
| Gender | |||
| Male | 81 (53.3) | ||
| Female | 71 (46.7) | ||
| Ethnicity | |||
| Malay | 84 (55.3) | ||
| Chinese | 50 (32.9) | ||
| Indian | 18 (11.8) | ||
| Education level | |||
| Primary | 42 (27.6) | ||
| Secondary | 71 (46.7) | ||
| Tertiary | 39 (25.7) | ||
| Marital status | |||
| Single | 16 (10.5) | ||
| Married | 136 (89.5) | ||
| Employment | |||
| Employed | 39 (25.7) | ||
| Unemployed | 113 (74.3) | ||
| Monthly income | |||
| ≤RM1000 | 75 (49.3) | ||
| >RM1000 | 77 (50.7) | ||
| Comorbidities a | |||
| Hypertension | 115 (75.7) | ||
| Diabetes mellitus | 53 (34.9) | ||
| Hyperlipidemia | 47 (30.9) | ||
| Others b | 41 (27.0) | ||
| No of comorbidities | |||
| None | 15 (9.9) | ||
| One | 51 (33.6) | ||
| Two | 47 (30.9) | ||
| ≥Three | 39 (25.7) | ||
| Dialysis vintage (months) | 56 (30.0–97.8) | 6–272 | |
| Dialysis adequacy (Kt/V) | 1.5 ± 0.3 c | 0.6–2.5 | |
| Adequate (≥1.2) | 128 (84.2) | ||
| Inadequate (<1.2) | 24 (15.8) |
| Variables | Model 1 | |||
|---|---|---|---|---|
| Block 1 | Block 2 | |||
| β | R2 | β | R2 | |
| Age | −0.395 *** | 0.394 | −0.199 * | 0.602 |
| Gender a | ||||
| Female | −0.374 *** | −0.090 | ||
| Ethnicity b | ||||
| Chinese | −0.058 | −0.050 | ||
| Indian | −0.189 * | −0.044 | ||
| Education level c | ||||
| Secondary | 0.047 | −0.014 | ||
| Tertiary | −0.023 | −0.028 | ||
| Marital status d | ||||
| Married | 0.039 | −0.007 | ||
| Employment e | ||||
| Unemployed | −0.079 | −0.054 | ||
| Monthly income f | ||||
| ≤RM 1000 | 0.060 | 0.051 | ||
| Clinical data | ||||
| No. of comorbidities | −0.101 | −0.079 | ||
| Dialysis vintage (months) | −0.154 * | −0.089 | ||
| Dialysis adequacy (Kt/V) | −0.011 | 0.049 | ||
| Body mass | ||||
| BMI (kg/m2) | - | 0.266 * | ||
| BF (%) | - | −0.334 *** | ||
| Unintentional weight loss (%) | - | 0.007 | ||
| Muscle mass | ||||
| MAMC (cm) | - | 0.111 | ||
| Serum creatinine (umol/L) | - | 0.229 ** | ||
| Serum chemistry | ||||
| Albumin (g/L) | - | 0.205 ** | ||
| Cholesterol (mmol/L) | - | 0.171 ** | ||
| Dietary intake | ||||
| DEI (kcal/kg BW/day) | - | 0.025 | ||
| DPI (g/kg BW/day) | - | 0.036 | ||
| PhA Cut-Off (°) | adjAUC | Sensitivity (%) | Specificity (%) | p-Value | |
|---|---|---|---|---|---|
| Overall (n = 152) | 4.11 | 0.746 | 62.5 | 61.7 | <0.001 |
| Male (n = 81) | 4.26 | 0.809 | 68.8 | 67.7 | <0.001 |
| Female (n = 71) | 3.30 | 0.719 | 68.8 | 85.5 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.-K.-M.; Lim, J.-H.; Ibrahim, I.; Chan, Y.-M.; Zakaria, N.F.; Yahya, R.; Daud, Z.A.M. Bioelectrical Impedance Analysis Derived-Phase Angle as a Pragmatic Tool to Detect Protein Energy Wasting among Multi-Ethnic Hemodialysis Patients. Diagnostics 2021, 11, 1745. https://doi.org/10.3390/diagnostics11101745
Lim C-K-M, Lim J-H, Ibrahim I, Chan Y-M, Zakaria NF, Yahya R, Daud ZAM. Bioelectrical Impedance Analysis Derived-Phase Angle as a Pragmatic Tool to Detect Protein Energy Wasting among Multi-Ethnic Hemodialysis Patients. Diagnostics. 2021; 11(10):1745. https://doi.org/10.3390/diagnostics11101745
Chicago/Turabian StyleLim, Cordelia-Kheng-May, Jun-Hao Lim, Imliya Ibrahim, Yoke-Mun Chan, Nor Fadhlina Zakaria, Rosnawati Yahya, and Zulfitri Azuan Mat Daud. 2021. "Bioelectrical Impedance Analysis Derived-Phase Angle as a Pragmatic Tool to Detect Protein Energy Wasting among Multi-Ethnic Hemodialysis Patients" Diagnostics 11, no. 10: 1745. https://doi.org/10.3390/diagnostics11101745
APA StyleLim, C.-K.-M., Lim, J.-H., Ibrahim, I., Chan, Y.-M., Zakaria, N. F., Yahya, R., & Daud, Z. A. M. (2021). Bioelectrical Impedance Analysis Derived-Phase Angle as a Pragmatic Tool to Detect Protein Energy Wasting among Multi-Ethnic Hemodialysis Patients. Diagnostics, 11(10), 1745. https://doi.org/10.3390/diagnostics11101745










