Altered Expression of Circadian Clock Genes in Patients with Atrial Fibrillation Is Associated with Atrial High-Rate Episodes and Left Atrial Remodeling
Abstract
1. Introduction
2. Materials and Methods
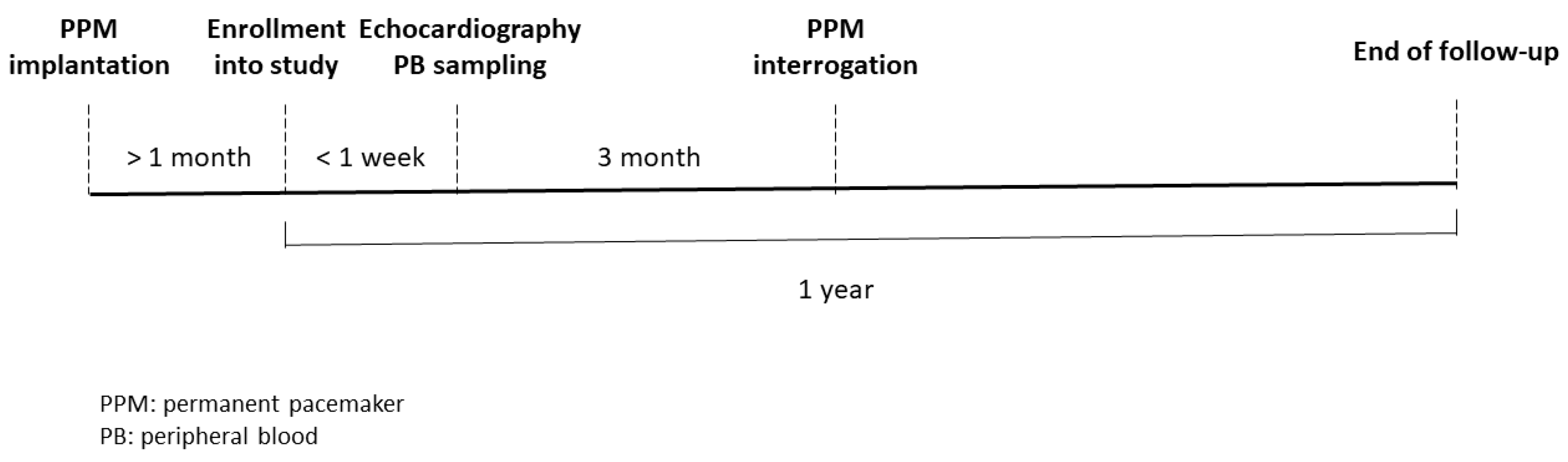
2.1. Patient Enrollment and Sample Management
2.2. Definition of AF Type and Atrial High-Rate Episodes and Measurement of Left Atrial (LA) Diameter and LA Volume
2.3. Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction Analysis (qRT-PCR) of Circadian Clock Genes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients with No AF, Paroxysmal AF, and Persistent AF
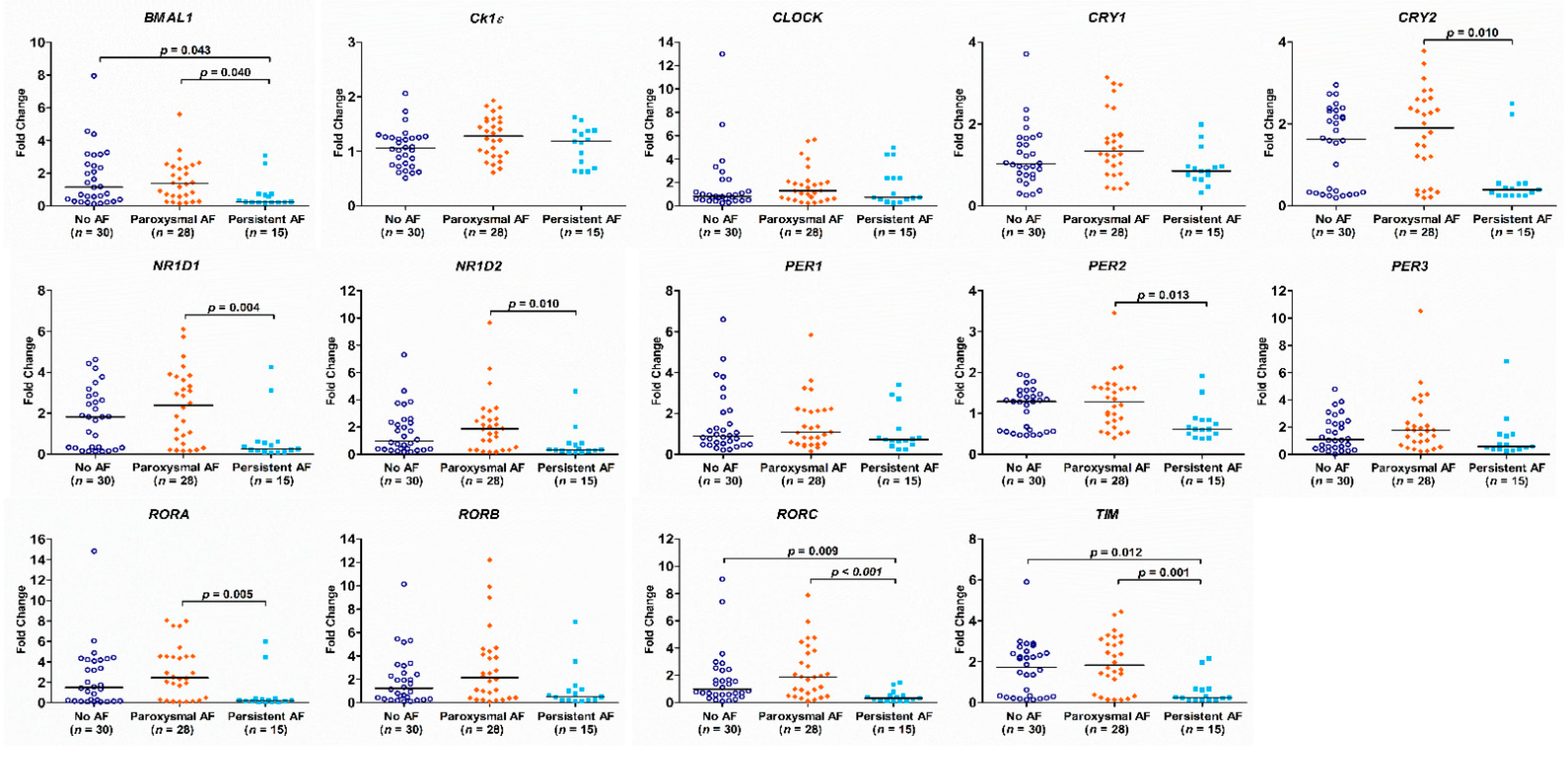
3.2. The Expression of the Circadian Clock Genes in Patients with No AF, Paroxysmal AF and Persistent AF
3.3. The Correlation of AHREs, LA Size, and the mRNA Expression of Circadian Clock Genes in Patients with AF
3.4. Multiple Linear Regression Model for Assessment of the Association between AHRE Burden and Other Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Developed with the Special Contribution of the European Heart Rhythm Association (EHRA); Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS); Authors/Task Force Members; Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar]
- Yamashita, T.; Murakawa, Y.; Sezaki, K.; Inoue, M.; Hayami, N.; Shuzui, Y.; Omata, M. Circadian variation of paroxysmal atrial fibrillation. Circulation 1997, 96, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Crnko, S.; Du Pre, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Woon, P.Y.; Kaisaki, P.J.; Braganca, J.; Bihoreau, M.T.; Levy, J.C.; Farrall, M.; Gauguier, D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 14412–14417. [Google Scholar] [CrossRef]
- Manfredini, R.; Boari, B.; Salmi, R.; Fabbian, F.; Pala, M.; Tiseo, R.; Portaluppi, F. Twenty-four-hour patterns in occurrence and pathophysiology of acute cardiovascular events and ischemic heart disease. Chronobiol. Int. 2013, 30, 6–16. [Google Scholar] [CrossRef]
- Lee, H.H.; Chen, Y.C.; Chen, J.J.; Lo, S.H.; Guo, Y.L.; Hu, H.Y. Insomnia and the risk of atrial fibrillation: A population-based cohort study. Acta Cardiol. Sin. 2017, 33, 165–172. [Google Scholar]
- Thomas, S.A.; Schuessler, R.B.; Berul, C.I.; Beardslee, M.A.; Beyer, E.C.; Mendelsohn, M.E.; Saffitz, J.E. Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction: Evidence for chamber-specific molecular determinants of conduction. Circulation 1998, 97, 686–691. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef]
- Jeyaraj, D.; Haldar, S.M.; Wan, X.; McCauley, M.D.; Ripperger, J.A.; Hu, K.; Lu, Y.; Eapen, B.L.; Sharma, N.; Ficker, E.; et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012, 483, 96–99. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. J. Cardiothorac. Surg. 2016, 18, 1609–1678. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Hwang, C.F.; Lin, P.M.; Chuang, J.H.; Hsu, C.M.; Lin, S.F.; Yang, M.Y. Sleep disturbance and altered expression of circadian clock genes in patients with sudden sensorineural hearing loss. Medicines (Baltimore) 2015, 94, e978. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Chang, J.G.; Lin, P.M.; Tang, K.P.; Chen, Y.H.; Lin, H.Y.; Liu, T.C.; Hsiao, H.H.; Liu, Y.C.; Lin, S.F. Downregulation of circadian clock genes in chronic myeloid leukemia: Alternative methylation pattern of hPER3. Cancer Sci. 2006, 97, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Pressman, G.; Caples, S.M.; Kanagala, R.; Gard, J.J.; Davison, D.E.; Malouf, J.F.; Ammash, N.M.; Friedman, P.A.; Somers, V.K. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004, 110, 364–367. [Google Scholar] [CrossRef]
- Deng, F.; Raza, A.; Guo, J. Treating obstructive sleep apnea with continuous positive airway pressure reduces risk of recurrent atrial fibrillation after catheter ablation: A meta-analysis. Sleep Med. 2018, 46, 5–11. [Google Scholar] [CrossRef]
- Yang, M.Y.; Lin, P.W.; Lin, H.C.; Lin, P.M.; Chen, I.Y.; Friedman, M.; Hung, C.F.; Salapatas, A.M.; Lin, M.C.; Lin, S.F. Alternations of circadian clock genes expression and oscillation in obstructive sleep apnea. J. Clin. Med. 2019, 8, 1634. [Google Scholar] [CrossRef]
- Kusanagi, H.; Hida, A.; Satoh, K.; Echizenya, M.; Shimizu, T.; Pendergast, J.S.; Yamazaki, S.; Mishima, K. Expression profiles of 10 circadian clock genes in human peripheral blood mononuclear cells. Neurosci. Res. 2008, 61, 136–142. [Google Scholar] [CrossRef]
- Hu, K.; Ivanov, P.; Hilton, M.F.; Chen, Z.; Ayers, R.T.; Stanley, H.E.; Shea, S.A. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 18223–18227. [Google Scholar] [CrossRef]
- Wang, N.; Yang, G.; Jia, Z.; Zhang, H.; Aoyagi, T.; Soodvilai, S.; Symons, J.D.; Schnermann, J.B.; Gonzalez, F.J.; Litwin, S.E.; et al. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008, 8, 482–491. [Google Scholar] [CrossRef]
- Viola, A.U.; James, L.M.; Archer, S.N.; Dijk, D.J. PER3 polymorphism and cardiac autonomic control: Effects of sleep debt and circadian phase. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2156–H2163. [Google Scholar] [CrossRef]
- Perkiomaki, J.; Ukkola, O.; Kiviniemi, A.; Tulppo, M.; Ylitalo, A.; Kesaniemi, Y.A.; Huikuri, H. Heart rate variability findings as a predictor of atrial fibrillation in middle-aged population. J. Cardiovasc. Electrophysiol. 2014, 25, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Vikman, S.; Makikallio, T.H.; Yli-Mayry, S.; Nurmi, M.; Airaksinen, K.E.; Huikuri, H.V. Heart rate variability and recurrence of atrial fibrillation after electrical cardioversion. Ann. Med. 2003, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W.; et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Guess, J.; Burch, J.B.; Ogoussan, K.; Armstead, C.A.; Zhang, H.; Wagner, S.; Hebert, J.R.; Wood, P.; Youngstedt, S.D.; Hofseth, L.J.; et al. Circadian disruption, Per3, and human cytokine secretion. Integr. Cancer Ther. 2009, 8, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Xu, B.; Xiang, Y.; Wu, L.; Zhang, Y.; Ma, X.; Tong, S.; Shu, M.; Song, Z.; Li, Y.; et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: A meta-analysis. Int. J. Cardiol. 2013, 169, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Dzeshka, M.S.; Lip, G.Y.; Snezhitskiy, V.; Shantsila, E. Cardiac fibrosis in patients with atrial fibrillation: Mechanisms and clinical implications. J. Am. Coll. Cardiol. 2015, 66, 943–959. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. J. Assoc. Am. Med. Coll. 2014, 311, 498–506. [Google Scholar] [CrossRef]
- Glotzer, T.V.; Hellkamp, A.S.; Zimmerman, J.; Sweeney, M.O.; Yee, R.; Marinchak, R.; Cook, J.; Paraschos, A.; Love, J.; Radoslovich, G.; et al. MOST Investigators. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003, 107, 1614–1619. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. ASSERT Investigators, Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012, 366, 120–129. [Google Scholar] [CrossRef]
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994, 89, 724–730. [Google Scholar] [CrossRef]
- Kumar, S.; The, A.W.; Medi, C.; Kistler, P.M.; Morton, J.B.; Kalman, J.M. Atrial remodeling in varying clinical substrates within beating human hearts: Relevance to atrial fibrillation. Prog. Biophys. Mol. Biol. 2012, 110, 278–294. [Google Scholar] [CrossRef]

| Variables | Persistent AF (n = 15) | Paroxysmal AF (n = 28) | No AF (n = 30) | p-Value |
|---|---|---|---|---|
| Age | 71.0 ± 8.3 | 71.0 ± 8.1 | 72.2 ± 8.7 | 0.840 |
| Sex (Male/Female) | 12/3 | 12/16 a | 9/21 a | 0.006 |
| Hypertension | 7 (46.7%) | 18 (64.3%) | 19 (63.3%) | 0.481 |
| Diabetes mellitus | 6 (40%) | 5 (17.9%) | 8 (26.7%) | 0.287 |
| Previous stroke | 3 (20%) | 5 (17.9%) | 2 (6.7%) | 0.338 |
| Heart failure | 1 (6.7%) | 5 (17.9%) | 2 (6.7%) | 0.330 |
| Coronary artery disease | 3 (20%) | 5 (17.9%) | 5 (16.7%) | 0.963 |
| Chronic kidney disease | 3 (20%) | 2 (7.1%) | 6 (20%) | 0.328 |
| Anxiety | 4 (26.7%) | 8 (28.6%) | 7 (23.3%) | 0.900 |
| Benzodiazepine | 2 (13.3%) | 2 (7.1%) | 4 (13.3%) | 0.713 |
| Non-benzodiazepine | 1 (6.7%) | 0 (0%) | 2 (6.7%) | 0.378 |
| Average heart rate | 74.5 ± 5.0 | 73.4 ± 7.9 | 71.9 ± 5.3 | 0.488 |
| AHRE burden (IQR) | 100 (100–100) | 0.5 (0–3.5) a | 0 (0–0) a | <0.001 |
| Echocardiographic data | ||||
| Left atrium diameter(mm) | 49.3 ± 9.3 | 40.8 ± 10.2 a | 38.9 ± 4.4 a | <0.001 |
| Left atrial volume (cm3) | 102.7 ± 37.5 | 62.4 ± 43.8 a | 50.7 ± 19.2 a | <0.001 |
| Aorta (mm) | 32.9 ± 5.1 | 32.1 ± 4.3 | 32.7 ± 4.4 | 0.802 |
| LVEDD (mm) | 51.1 ± 8.3 | 47.4 ± 5.6 | 48.4 ± 8.3 | 0.294 |
| LVESD (mm) | 35.1 ± 9.4 | 30.4 ± 4.3 | 30.8 ± 7.5 | 0.089 |
| LVEF (%) | 60.0 ± 10.9 | 65.1 ± 7.6 | 65.9 ± 9.2 | 0.106 |
| Septal E/e’ ratio | 16.3 ± 9.5 | 13.9 ± 8.9 | 14.2 ± 9.3 | 0.740 |
| DT (ms) | 181.2 ± 64.6 | 224.6 ± 72.7 | 196.7 ± 44.2 | 0.097 |
| PAP (mmHg) | 25.2 ± 10.8 | 24.9 ± 9.2 | 24.7 ± 8.4 | 0.984 |
| Variables | r | p-Value |
|---|---|---|
| Left atrial diameter | 0.593 | <0.001 |
| Left atrial volume | 0.651 | <0.001 |
| BMAL1 | −0.452 | 0.002 |
| CK1ε | −0.244 | 0.115 |
| CLOCK | 0.005 | 0.973 |
| CRY1 | −0.329 | 0.031 |
| CRY2 | −0.516 | <0.001 |
| NR1D1 | −0.518 | <0.001 |
| NR1D2 | −0.410 | 0.006 |
| PER1 | −0.216 | 0.163 |
| PER2 | −0.473 | 0.001 |
| PER3 | −0.306 | 0.046 |
| RORA | −0.508 | 0.001 |
| RORB | −0.424 | 0.005 |
| RORC | −0.569 | <0.001 |
| TIM | −0.531 | <0.001 |
| Variables | Standardized β Coefficient | p-Value |
|---|---|---|
| Male sex | 0.188 | 0.066 |
| Age | 0.139 | 0.127 |
| Left atrial volume | 0.608 | <0.001 |
| BMAL1 | 0.385 | 0.050 |
| CRY1 | 0.386 | 0.041 |
| CRY2 | −0.528 | 0.382 |
| NR1D1 | 1.149 | 0.016 |
| NR1D2 | 0.248 | 0.569 |
| PER2 | −0.128 | 0.687 |
| PER3 | 0.148 | 0.371 |
| RORA | −1.676 | 0.025 |
| RORB | 0.257 | 0.112 |
| RORC | −0.470 | 0.064 |
| TIM | 0.265 | 0.520 |
| Variables | Tertile 1 | Tertile 2 | Tertile 3 | p-Value for Linear Trend |
|---|---|---|---|---|
| CRY1a | 1 (0–27.5) | 2.5 (0–97.8) | 100 (1–100) | 0.047 |
| NR1D1b | 0 (0–14) | 11.1 (0–100) | 98.5 (1–100) | 0.010 |
| RORAc | 1 (0–14) | 2.5 (0–87.3) | 100 (1–100) | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Chuang, J.-H.; Wang, H.-T.; Chen, H.-C.; Liu, W.-H.; Yang, M.-Y. Altered Expression of Circadian Clock Genes in Patients with Atrial Fibrillation Is Associated with Atrial High-Rate Episodes and Left Atrial Remodeling. Diagnostics 2021, 11, 90. https://doi.org/10.3390/diagnostics11010090
Chen Y-L, Chuang J-H, Wang H-T, Chen H-C, Liu W-H, Yang M-Y. Altered Expression of Circadian Clock Genes in Patients with Atrial Fibrillation Is Associated with Atrial High-Rate Episodes and Left Atrial Remodeling. Diagnostics. 2021; 11(1):90. https://doi.org/10.3390/diagnostics11010090
Chicago/Turabian StyleChen, Yung-Lung, Jiin-Haur Chuang, Hui-Ting Wang, Huang-Chung Chen, Wen-Hao Liu, and Ming-Yu Yang. 2021. "Altered Expression of Circadian Clock Genes in Patients with Atrial Fibrillation Is Associated with Atrial High-Rate Episodes and Left Atrial Remodeling" Diagnostics 11, no. 1: 90. https://doi.org/10.3390/diagnostics11010090
APA StyleChen, Y.-L., Chuang, J.-H., Wang, H.-T., Chen, H.-C., Liu, W.-H., & Yang, M.-Y. (2021). Altered Expression of Circadian Clock Genes in Patients with Atrial Fibrillation Is Associated with Atrial High-Rate Episodes and Left Atrial Remodeling. Diagnostics, 11(1), 90. https://doi.org/10.3390/diagnostics11010090





