Tear ATG5 as a Potential Novel Biomarker in the Diagnosis of Sjögren Syndrome
Abstract
1. Introduction
2. Materials and Methods
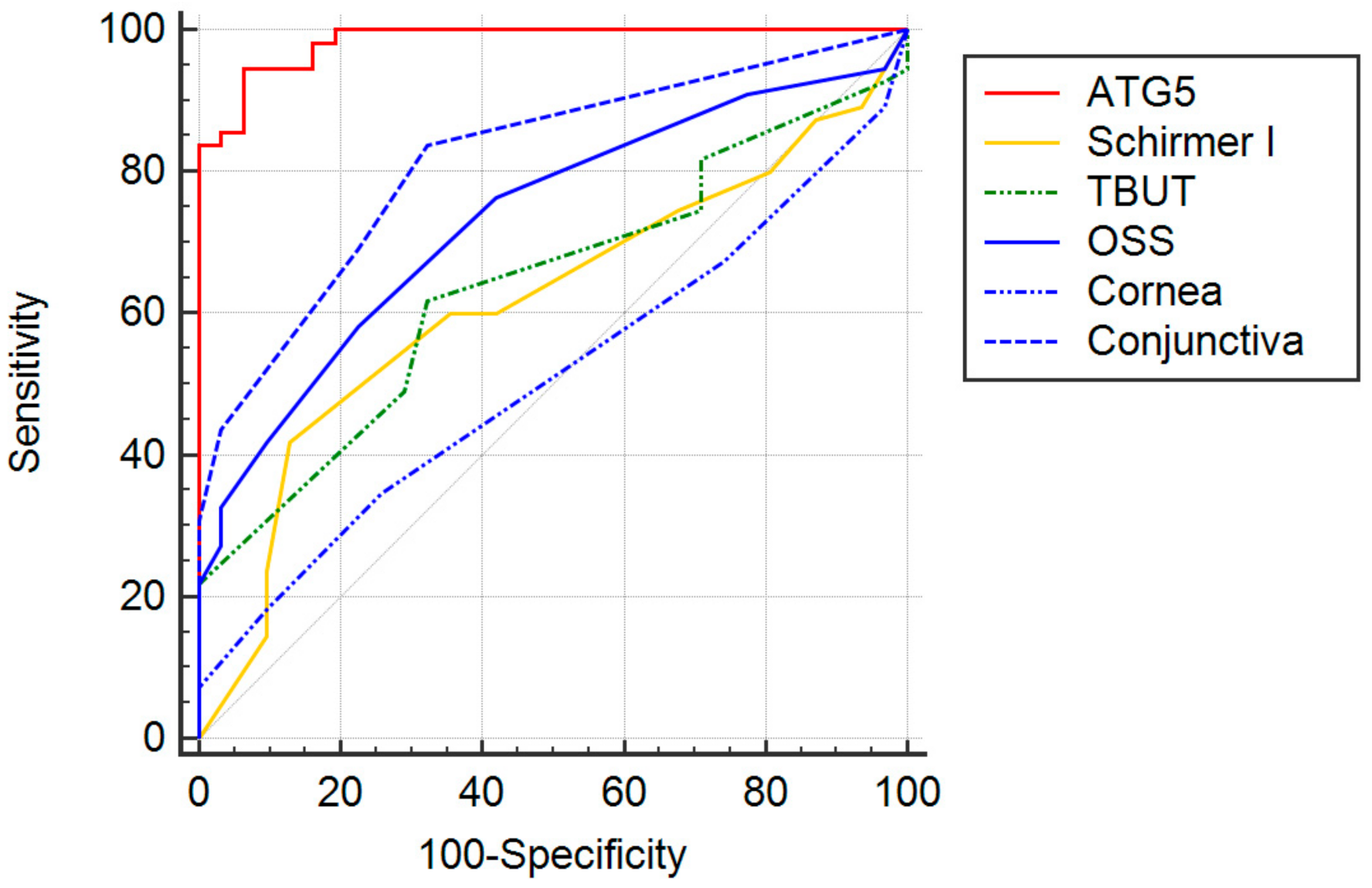
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tzioufas, A.G.; Wassmuth, R.; Dafni, U.G.; Guialis, A.; Haga, H.J.; Isenberg, D.A.; Jonsson, R.; Kalden, J.R.; Kiener, H.; Sakarellos, C.; et al. Clinical, immunological, and immunogenetic aspects of autoantibody production against Ro/SSA, La/SSB and their linear epitopes in primary Sjogren’s syndrome (pSS): A European multicentre study. Ann. Rheum. Dis. 2002, 61, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, A.H.; Potts, R.; Schmid, W.R.; Scofield, R.H.; Harley, J.B. The genetics of primary Sjögren’s syndrome. Curr. Rheumatol. Rep. 2003, 5, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Bathke, M.; Lin, H.H.; Chibly, A.M.; Zhang, W.; Sun, X.; Chen, C.H.; Flodby, P.; Borok, Z.; Wu, R.; Arnett, D.; et al. Deletion of ATG5 shows a role of autophagy in salivary homeostatic control. J. Dent Res. 2013, 92, 911–917. [Google Scholar] [CrossRef]
- Katsiougiannis, S.; Tenta, R.; Skopouli, F.N. Endoplasmic reticulum stress causes autophagy and apoptosis leading to cellular redistribution of the autoantigens Ro/Sjogren’s syndrome-related antigen A (SSA) and La/SSB in salivary gland epithelial cells. Clin. Exp. Immunol. 2015, 181, 244–252. [Google Scholar] [CrossRef]
- Seo, Y.; Ji, Y.W.; Lee, S.M.; Shim, J.; Noh, H.; Yeo, A.; Park, C.; Park, M.S.; Chang, E.J.; Lee, H.K. Activation of HIF-1alpha (hypoxia inducible factor-1alpha) prevents dry eye-induced acinar cell death in the lacrimal gland. Cell Death Dis. 2014, 5, e1309. [Google Scholar] [CrossRef]
- Zhou, X.J.; Zhang, H. Autophagy in immunity: Implications in etiology of autoimmune/autoinflammatory diseases. Autophagy 2012, 8, 1286–1299. [Google Scholar] [CrossRef]
- Casciola-Rosen, L.A.; Anhalt, G.; Rosen, A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994, 179, 1317–1330. [Google Scholar] [CrossRef]
- Okuma, A.; Hoshino, K.; Ohba, T.; Fukushi, S.; Aiba, S.; Akira, S.; Ono, M.; Kaisho, T.; Muta, T. Enhanced apoptosis by disruption of the STAT3-IkappaB-zeta signaling pathway in epithelial cells induces Sjogren’s syndrome-like autoimmune disease. Immunity 2013, 38, 450–460. [Google Scholar] [CrossRef]
- Hu, S.; Vissink, A.; Arellano, M.; Roozendaal, C.; Zhou, H.; Kallenberg, C.G.; Wong, D.T. Identification of autoantibody biomarkers for primary Sjogren’s syndrome using protein microarrays. Proteomics 2011, 11, 1499–1507. [Google Scholar] [CrossRef]
- Byun, Y.S.; Lee, H.J.; Shin, S.; Chung, S.H. Elevation of autophagy markers in Sjogren syndrome dry eye. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Shiboski, C.H.; Shiboski, S.C.; Heidenreich, A.M.; Kitagawa, K.; Zhang, S.; Hamann, S.; Larkin, G.; McNamara, N.A.; Greenspan, J.S.; et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren’s Syndrome International Registry. Am. J. Ophthalmol. 2010, 149, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Tsubota, K.; Kaido, M.; Yagi, Y.; Fujihara, T.; Shimmura, S. Diseases associated with ocular surface abnormalities: The importance of reflex tearing. Br. J. Ophthalmol. 1999, 83, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Bitton, E.; Wittich, W. Influence of eye position on the Schirmer tear test. Cont. Lens Anterior Eye 2014, 37, 257–261. [Google Scholar] [CrossRef]
- Tomlinson, A.; Khanal, S.; Ramaesh, K.; Diaper, C.; McFadyen, A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4309–4315. [Google Scholar] [CrossRef]
- Akpek, E.K.; Wu, H.Y.; Karakus, S.; Zhang, Q.; Masli, S. Differential Diagnosis of Sjögren Versus Non-Sjögren Dry Eye through Tear Film Biomarkers. Cornea 2020, 39, 991–997. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Posa, A.; Brauer, L.; Schicht, M.; Garreis, F.; Beileke, S.; Paulsen, F. Schirmer strip vs. capillary tube method: Non-invasive methods of obtaining proteins from tear fluid. Ann. Anat. 2013, 195, 137–142. [Google Scholar] [CrossRef]
- Lim, S.A.; Nam, D.H.; Lee, J.H.; Kwok, S.K.; Park, S.H.; Chung, S.H. Association of IL-21 cytokine with severity of primary Sjogren syndrome dry eye. Cornea 2015, 34, 248–252. [Google Scholar] [CrossRef]
- Yang, S.; Lee, H.J.; Kim, D.Y.; Shin, S.; Barabino, S.; Chung, S.H. The Use of Conjunctival Staining to Measure Ocular Surface Inflammation in Patients with Dry Eye. Cornea 2019, 38, 698–705. [Google Scholar] [CrossRef]
- Versura, P.; Frigato, M.; Cellini, M.; Mulè, R.; Malavolta, N.; Campos, E.C. Diagnostic performance of tear function tests in Sjogren’s syndrome patients. Eye 2007, 21, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Tomosugi, N.; Kitagawa, K.; Takahashi, N.; Sugai, S.; Ishikawa, I. Diagnostic potential of tear proteomic patterns in Sjogren’s syndrome. J. Proteome. Res. 2005, 4, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Hamm-Alvarez, S.F.; Janga, S.R.; Edman, M.C.; Madrigal, S.; Shah, M.; Frousiakis, S.E.; Renduchintala, K.; Zhu, J.; Bricel, S.; Silka, K.; et al. Tear cathepsin S as a candidate biomarker for Sjogren’s syndrome. Arthriti. Rheumat. 2014, 66, 1872–1881. [Google Scholar] [CrossRef]
- Shinzawa, M.; Dogru, M.; Den, S.; Ichijima, T.; Higa, K.; Kojima, T.; Seta, N.; Nomura, T.; Tsubota, K.; Shimazaki, J. Epidermal fatty acid-binding protein: A novel marker in the diagnosis of dry eye disease in sjögren syndrome. Int. J. Mol. Sci. 2018, 19, 3463. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T.; Fang, P.C.; Chao, T.L.; Chen, A.; Lai, Y.H.; Huang, Y.T.; Tseng, C.Y. Tear proteomics approach to monitoring Sjögren syndrome or dry eye disease. Int. J. Mol. Sci. 2019, 20, 1932. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Khalil, M.; Ravetch, J.; Diamond, B. The naive B cell repertoire predisposes to antigen-induced systemic lupus erythematosus. J. Immunol. 2003, 170, 4826–4832. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Thomson, B.P.; Remmers, E.F.; Eyre, S.; Hinks, A.; Guiducci, C.; Catanese, J.J.; Xie, G.; Stahl, E.A.; Chen, R.; et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat. Genet. 2009, 41, 1313–1318. [Google Scholar] [CrossRef]
| Parameters | Non-SS DE 1 | SS DE 2 | p-Value | |
|---|---|---|---|---|
| Number of patients (eyes) | 31 | 55 | N/A | |
| Sex | Male:Female | 2:29 | 1:54 | N/A |
| Age | Mean ± SD | 55.7 ± 11.1 | 50.7 ± 10.6 | 0.0311 |
| Median, range | 57, 21 to 77 | 51, 32 to 69 | ||
| Schirmer I | Mean ± SD | 4.7 ± 2.5 | 3.7 ± 3.0 | 0.0721 |
| Median, range | 5, 0 to 10 | 3, 0 to 10 | ||
| TBUT 3 | Mean ± SD | 4.0 ± 1.6 | 3.0 ± 2.3 | 0.0286 |
| Median, range | 4, 2 to 7 | 3, 0 to 8 | ||
| OSS 4 | Mean ± SD | 2.5 ± 1.5 | 4.7 ± 3.0 | 0.0003 |
| Median, range | 2, 0 to 7 | 4, 0 to 11 | ||
| Corneal staining | Mean ± SD | 2.1 ± 1.0 | 2.2 ± 1.4 | 0.8996 |
| Median, range | 2, 0 to 4 | 2, 0 to 5 | ||
| Conjunctival staining | Mean ± SD | 0.6 ± 0.9 | 2.6 ± 1.9 | <0.0001 |
| Median, range | 0, 0 to 3 | 2, 0 to 6 | ||
| OSDI 5 | Mean ± SD | 30.4 ± 2.1 | 38.8 ± 20.6 | 0.0916 |
| Median, range | 30.0, 56.8 | 36.6, 0 to 77 | ||
| Tear ATG5 | Mean ± SD | 2.48 ± 1.25 | 11.21 ± 6.06 | <0.0001 |
| Median, range | 2.58, 0.30 to 5.33 | 9.64, 3.47 to 25.30 | ||
| Spearman’s Rho | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Schirmer I | −0.08747 | −0.3519 to 0.1899 | 0.5254 |
| TBUT 1 | −0.08178 | −0.3469 to 0.1954 | 0.5528 |
| OSS 2 score | 0.3898 | 0.1309 to 0.5989 | 0.0033 |
| Corneal score | 0.2833 | 0.01132 to 0.5162 | 0.0361 |
| Conjunctival score | 0.3687 | 0.1066 to 0.5829 | 0.0056 |
| OSDI 3 score | 0.2087 | −0.06801 to 0.4556 | 0.1263 |
| Spearman’s Rho | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Schirmer I | −0.1797 | −0.5103 to 0.1972 | 0.3334 |
| TBUT 1 | −0.02740 | −0.3875 to 0.3400 | 0.8837 |
| OSS 2 score | −0.1460 | −0.4843 to 0.2302 | 0.4331 |
| Corneal score | −0.1141 | −0.4590 to 0.2607 | 0.5412 |
| Conjunctival score | −0.07130 | −0.4243 to 0.3005 | 0.7031 |
| OSDI 3 score | 0.1143 | −0.2605 to 0.4591 | 0.5405 |
| Tests | Cutoff | Sensitivity (95% CI 2) | Specificity (95% CI 2) | Likelihood Ratio (95% CI 2) |
|---|---|---|---|---|
| Schirmer I | ≤5 mm/5 min 3 | 74.6 (61.0 to 85.3) | 32.3 (16.7 to 51.4) | 1.1 (0.8 to 1.2) |
| TBUT | ≤4 s | 61.8 (47.7 to 74.6) | 67.7 (48.6 to 83.3) | 1.92 (1.1 to 3.3) |
| OSS 1 | ≥5 3 | 32.7 (20.7 to 46.7) | 96.8 (83.3 to 99.9) | 10.2 (1.4 to 72.4) |
| OSS 1 | ≥3 4 | 58.2 (44.1 to 71.3) | 77.4 (58.9 to 90.4) | 2.6 (1.3 to 5.1) |
| ATG5 | >4.0 ng/mL/μg | 94.6 (84.9 to 98.9) | 93.6 (78.6 to 99.2) | 14.7 (3.8 to 56.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, Y.-S.; Lee, H.J.; Shin, S.; Choi, M.Y.; Kim, H.-S.; Chung, S.-H. Tear ATG5 as a Potential Novel Biomarker in the Diagnosis of Sjögren Syndrome. Diagnostics 2021, 11, 71. https://doi.org/10.3390/diagnostics11010071
Byun Y-S, Lee HJ, Shin S, Choi MY, Kim H-S, Chung S-H. Tear ATG5 as a Potential Novel Biomarker in the Diagnosis of Sjögren Syndrome. Diagnostics. 2021; 11(1):71. https://doi.org/10.3390/diagnostics11010071
Chicago/Turabian StyleByun, Yong-Soo, Hyun Jung Lee, Soojung Shin, Moon Young Choi, Hyung-Seung Kim, and So-Hyang Chung. 2021. "Tear ATG5 as a Potential Novel Biomarker in the Diagnosis of Sjögren Syndrome" Diagnostics 11, no. 1: 71. https://doi.org/10.3390/diagnostics11010071
APA StyleByun, Y.-S., Lee, H. J., Shin, S., Choi, M. Y., Kim, H.-S., & Chung, S.-H. (2021). Tear ATG5 as a Potential Novel Biomarker in the Diagnosis of Sjögren Syndrome. Diagnostics, 11(1), 71. https://doi.org/10.3390/diagnostics11010071





