Changes of Fixed Anatomical Spinopelvic Parameter in Patients with Lumbosacral Transitional Vertebrae: A Matched Pair Analysis
Abstract
1. Introduction
2. Materials and Methods
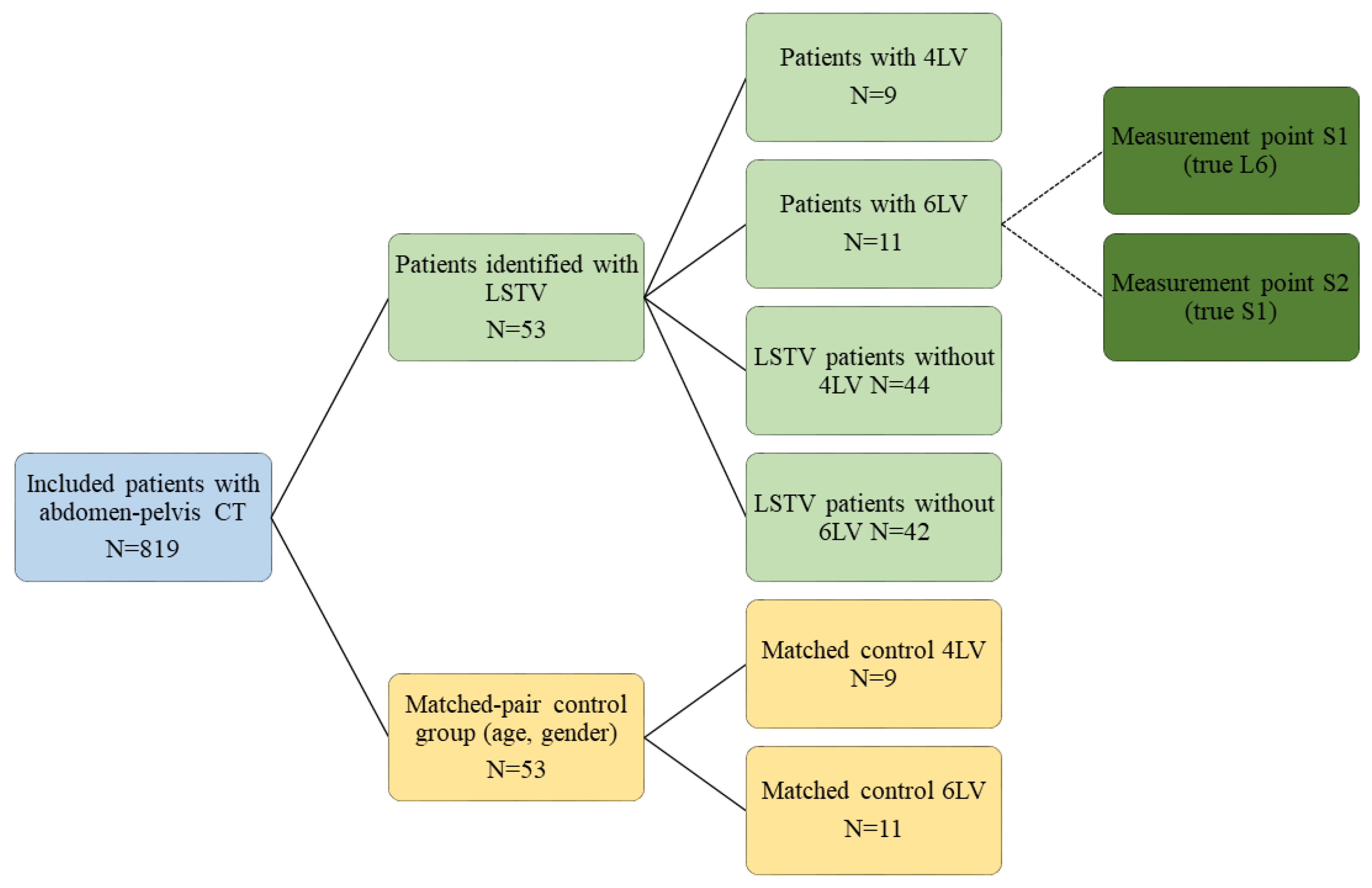
2.1. Individuals
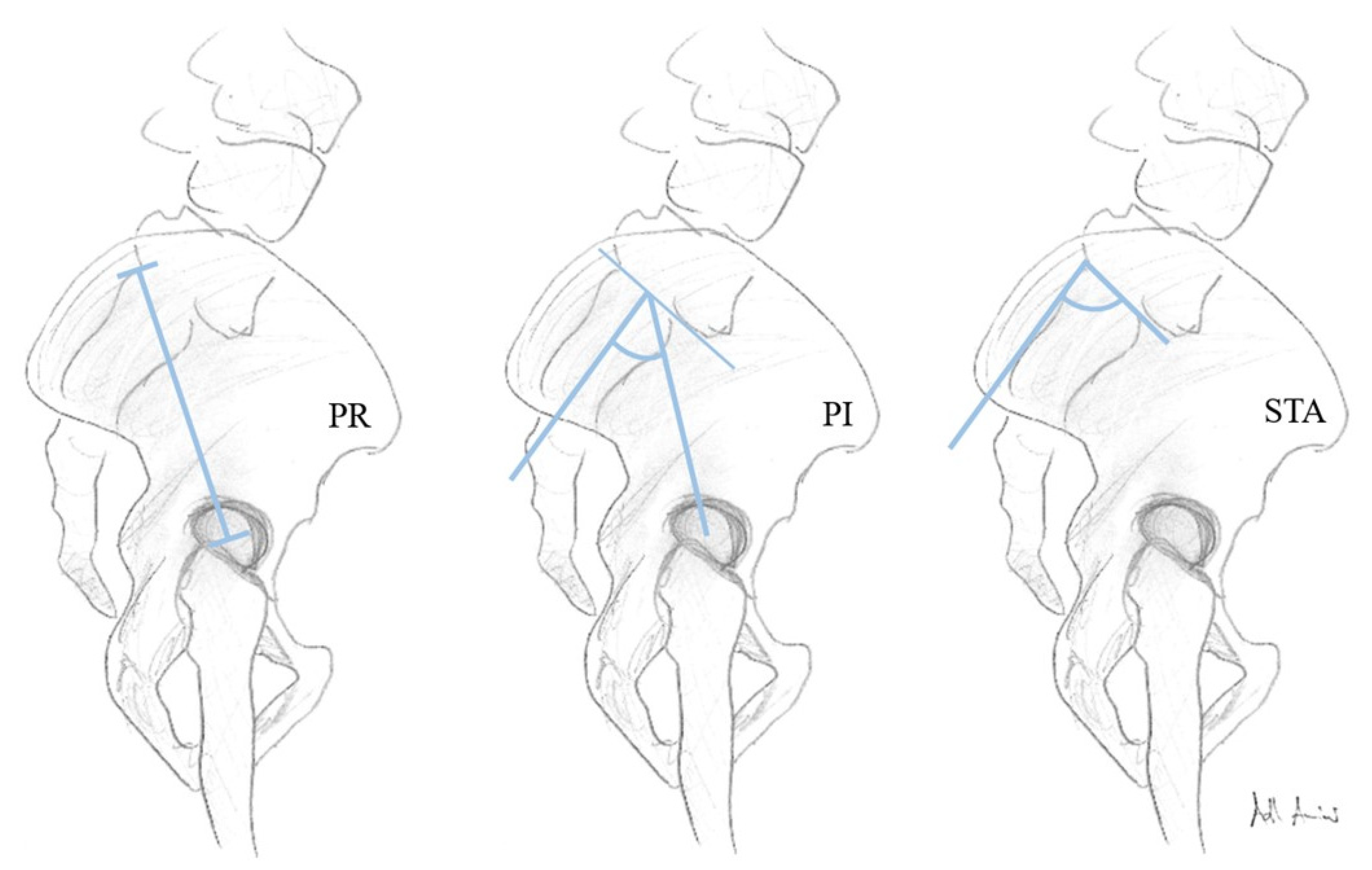
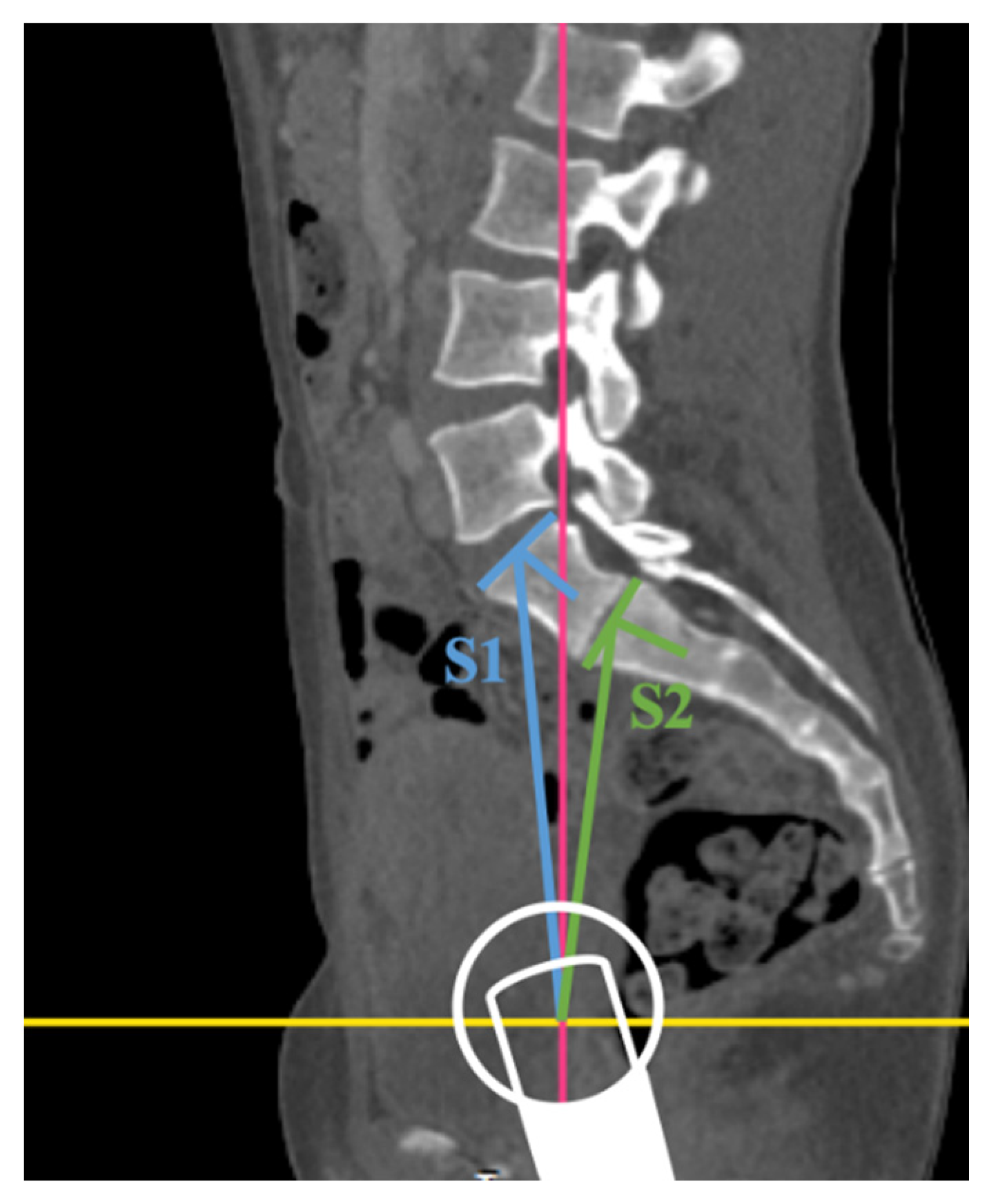
2.2. Image Assessment
2.3. Statistical Analysis
3. Results
3.1. Patients
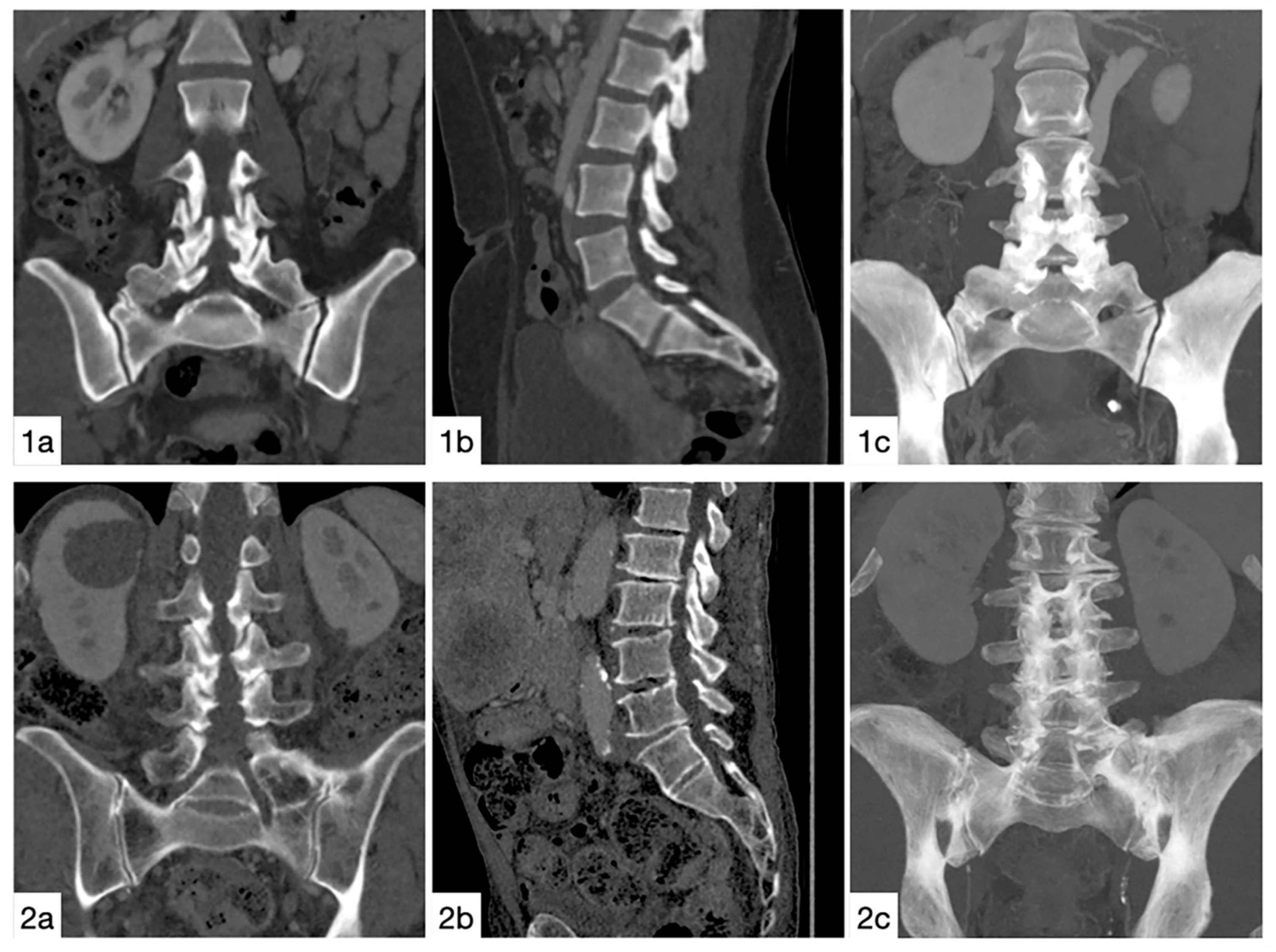
3.2. Prevalence
3.3. Lumbosacral Transitional Vertebrae Versus Control Group
3.4. Six Lumbar Vertebrae
3.5. Four Lumbar Vertebrae
3.6. Fixed Anatomical Spinopelvic Parameter Pending the Degree of LSTV Expression
3.7. Accuracy of the Radiographic Measurement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apazidis, A.; Ricart, P.A.; Diefenbach, C.M.; Spivak, J.M. The prevalence of transitional vertebrae in the lumbar spine. Spine J. 2011, 11, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Yang, X.F.; Yang, S.W.; Han, P. Lumbosacral transitional vertebra in a population-based study of 5860 individuals: Prevalence and relationship to low back pain. Eur. J. Radiol. 2014, 83, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.K. Morphological traits in sacra associated with complete and partial lumbarization of first sacral segment. Spine J. 2010, 10, 910–915. [Google Scholar] [CrossRef]
- Castellvi, A.E.; Goldstein, L.A.; Chan, D.P. Lumbosacral transitional vertebrae and their relationship with lumbar extradural defects. Spine (Phila Pa 1976) 1984, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Tokgoz, N.; Ucar, M.; Erdogan, A.B.; Kilic, K.; Ozcan, C. Are spinal or paraspinal anatomic markers helpful for vertebral numbering and diagnosing lumbosacral transitional vertebrae? Korean, J. Radiol. 2014, 15, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Usui, A.; Hosokai, Y.; Kawasumi, Y.; Abiko, K.; Funayama, M.; Saito, H. The prevalence of morphological changes in the thoracolumbar spine on whole-spine computed tomographic images. Insights Imaging 2014, 5, 77–83. [Google Scholar] [CrossRef]
- Paik, N.C.; Lim, C.S.; Jang, H.S. Numeric and morphological verification of lumbosacral segments in 8280 consecutive patients. Spine (Phila Pa 1976) 2013, 38, E573–E578. [Google Scholar] [CrossRef]
- Yokoyama, K.; Kawanishi, M.; Yamada, M.; Tanaka, H.; Ito, Y.; Kawabata, S.; Kuroiwa, T. Spinopelvic alignment and sagittal balance of asymptomatic adults with 6 lumbar vertebrae. Eur. Spine J. 2016, 25, 3583–3588. [Google Scholar] [CrossRef]
- Džupa, V.; Slepanek, M.; Striz, M.; Krbec, M.; Chmelova, J.; Kachlik, D.; Baca, V. Developmental malformations in the area of the lumbosacral transitional vertebrae and sacrum: Differences in gender and left/right distribution. Surg. Radiol. Anat. 2014, 36, 689–693. [Google Scholar] [CrossRef]
- Arima, H.; Dimar, J.R.; Glassman, S.D.; Yamato, Y.; Matsuyama, Y.; Mac-Thiong, J.M.; Roussouly, P.; Cook, B.; Carreon, L.Y. Differences in lumbar and pelvic parameters among African American, Caucasian and Asian populations. Eur. Spine J. 2018, 27, 2990–2998. [Google Scholar] [CrossRef]
- de Bruin, F.; Ter Horst, S.; Bloem, J.L.; van den Berg, R.; de Hooge, M.; van Gaalen, F.; Dagfinrud, H.; van Oosterhout, M.; van der Heijde, D.; Reijnierse, M.; et al. Prevalence and clinical significance of lumbosacral transitional vertebra (LSTV) in a young back pain population with suspected axial spondyloarthritis: Results of the SPondyloArthritis Caught Early (SPACE) cohort. Skelet. Radiol. 2017, 46, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Sekharappa, V.; Amritanand, R.; Krishnan, V.; David, K.S. Lumbosacral transition vertebra: Prevalence and its significance. Asian Spine J. 2014, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Boulay, C.; Tardieu, C.; Hecquet, J.; Benaim, C.; Mouilleseaux, B.; Marty, C.; Prat-Pradal, D.; Legaye, J.; Duval-Beaupère, G.; Pélissier, J. Sagittal alignment of spine and pelvis regulated by pelvic incidence: Standard values and prediction of lordosis. Eur. Spine J. 2006, 15, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Legaye, J.; Marty, C.; Hecquet, J. Pelvic incidence: A fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur. Spine J. 1998, 7, 99–103. [Google Scholar] [CrossRef]
- Lafage, V.; Schwab, F.; Patel, A.; Hawkinson, N.; Farcy, J.P. Pelvic tilt and truncal inclination: Two key radiographic parameters in the setting of adults with spinal deformity. Spine (Phila Pa 1976) 2009, 34, E599–606. [Google Scholar] [CrossRef]
- Roussouly, P.; Gollogly, S.; Berthonnaud, E.; Dimnet, J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine (Phila Pa 1976) 2005, 30, 346–353. [Google Scholar] [CrossRef]
- Laouissat, F.; Sebaaly, A.; Gehrchen, M.; Roussouly, P. Classification of normal sagittal spine alignment: Refounding the Roussouly classification. Eur. Spine J. 2018, 27, 2002–2011. [Google Scholar] [CrossRef]
- Kumar, M.; Baklanov, A.; Chopin, D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur. Spine J. 2001, 10, 314–319. [Google Scholar] [CrossRef]
- Schwab, F.; Farcy, J.-P.; Bridwell, K.; Berven, S.; Glassman, S.; Harrast, J.; Horton, W. AA clinical impact classification of scoliosis in the adult. Spine (Phila Pa 1976) 2006, 31, 2109–2114. [Google Scholar] [CrossRef]
- Schwab, F.; Ungar, B.; Blondel, B.; Buchowski, J.; Coe, J.; Deinlein, D. Scoliosis Research Society-Schwab adult spinal deformity classification: A validation study. Spine (Phila Pa 1976) 2012, 37, 1077–1082. [Google Scholar] [CrossRef]
- Yilgor, C.; Sogunmez, N.; Boissiere, L.; Yavuz, Y.; Obeid, I.; Kleinstück, F. Global Alignment and Proportion (GAP) Score: Development and Validation of a New Method of Analyzing Spinopelvic Alignment to Predict Mechanical Complications After Adult Spinal Deformity Surgery. J. Bone Jt. Surg. Am. 2017, 99, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Dreischarf, M.; Pries, E.; Bashkuev, M.; Putzier, M.; Schmidt, H. Differences between clinical "snap-shot" and "real-life" assessments of lumbar spine alignment and motion-What is the "real" lumbar lordosis of a human being? J. Biomech. 2016, 49, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Barrey, C.; Jund, J.; Perrin, G.; Roussouly, P. Spinopelvic alignment of patients with degenerative spondylolisthesis. Neurosurgery 2007, 61, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Labelle, H.; Roussouly, P.; Berthonnaud, E.; Transfeldt, E.; O’Brien, M.; Chopin, D. Spondylolisthesis, pelvic incidence, and spinopelvic balance: A correlation study. Spine (Phila Pa 1976) 2004, 29, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Strube, P.; Pumberger, M.; Sonnow, L.; Zippelius, T.; Nowack, D.; Zahn, R.K. Association Between Lumbar Spinal Degeneration and Anatomic Pelvic Parameters. Clin. Spine Surg. 2018, 31, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Abola, M.V.; Teplensky, J.R.; Cooperman, D.R.; Bauer, J.M.; Liu, R.W. Pelvic Incidence in Spines With 4 and 6 Lumbar Vertebrae. Glob. Spine J. 2019, 9, 708–712. [Google Scholar] [CrossRef]
- Price, R.; Okamoto, M.; Le Huec, J.; Hasegawa, K. Normative spino-pelvic parameters in patients with the lumbarization of S1 compared to a normal asymptomatic population. Eur. Spine J. 2016, 25, 3694–3698. [Google Scholar] [CrossRef]
- Kyrölä, K.; Kautiainen, H.; Ylinen, J.; Lehtola, R.; Kiviranta, I.; Hakkinen, A. Spinopelvic Parameters and Sagittal Alignment of Symptomatic Degenerative Adult Spinal Disorder Patients With 6 Lumbar Vertebrae. Clin. Spine Surg. 2019, 32, E43–E49. [Google Scholar] [CrossRef]
- Schwab, F.; Patel, A.; Ungar, B.; Farcy, J.P.; Lafage, V. Adult spinal deformity-postoperative standing imbalance: How much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976) 2010, 35, 2224–2231. [Google Scholar] [CrossRef]
- Glassman, S.D.; Bridwell, K.; Dimar, J.R.; Horton, W.; Berven, S.; Schwab, F. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005, 30, 2024–2029. [Google Scholar] [CrossRef]
- Mehta, V.A.; Amin, A.; Omeis, I.; Gokaslan, Z.L.; Gottfried, O.N. Implications of spinopelvic alignment for the spine surgeon. Neurosurgery 2015, 76 (Suppl. 1), S42–56. [Google Scholar] [CrossRef] [PubMed]
- Le Huec, J.C.; Thompson, W.; Mohsinaly, Y.; Barrey, C.; Faundez, A. Sagittal balance of the spine. Eur. Spine J. 2019, 28, 1889–1905. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.B.; Levin, A.; Burd, T.; Longley, M. Corrective osteotomies in spine surgery. J. Bone Jt. Surg. Am. 2008, 90, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Le Huec, J.-C.; Hasegawa, K. Normative values for the spine shape parameters using 3D standing analysis from a database of 268 asymptomatic Caucasian and Japanese subjects. Eur. Spine J. 2016, 25, 3630–3637. [Google Scholar] [CrossRef]
- Khalsa, A.S.; Mundis, G.M., Jr.; Yagi, M.; Fessler, R.G.; Bess, S.; Hosogane, N. Variability in Assessing Spinopelvic Parameters With Lumbosacral Transitional Vertebrae: Inter-and Intraobserver Reliability Among Spine Surgeons. Spine (Phila Pa 1976) 2018, 43, 813–816. [Google Scholar] [CrossRef]
- Konin, G.P.; Walz, D.M. Lumbosacral transitional vertebrae: Classification, imaging findings, and clinical relevance. AJNR Am. J. Neuroradiol. 2010, 31, 1778–1786. [Google Scholar] [CrossRef]
- Aly, I.; Chapman, J.R.; Oskouian, R.J.; Loukas, M.; Tubbs, R.S. Lumbar ribs: A comprehensive review. Childs Nerv. Syst. 2016, 32, 781–785. [Google Scholar] [CrossRef]
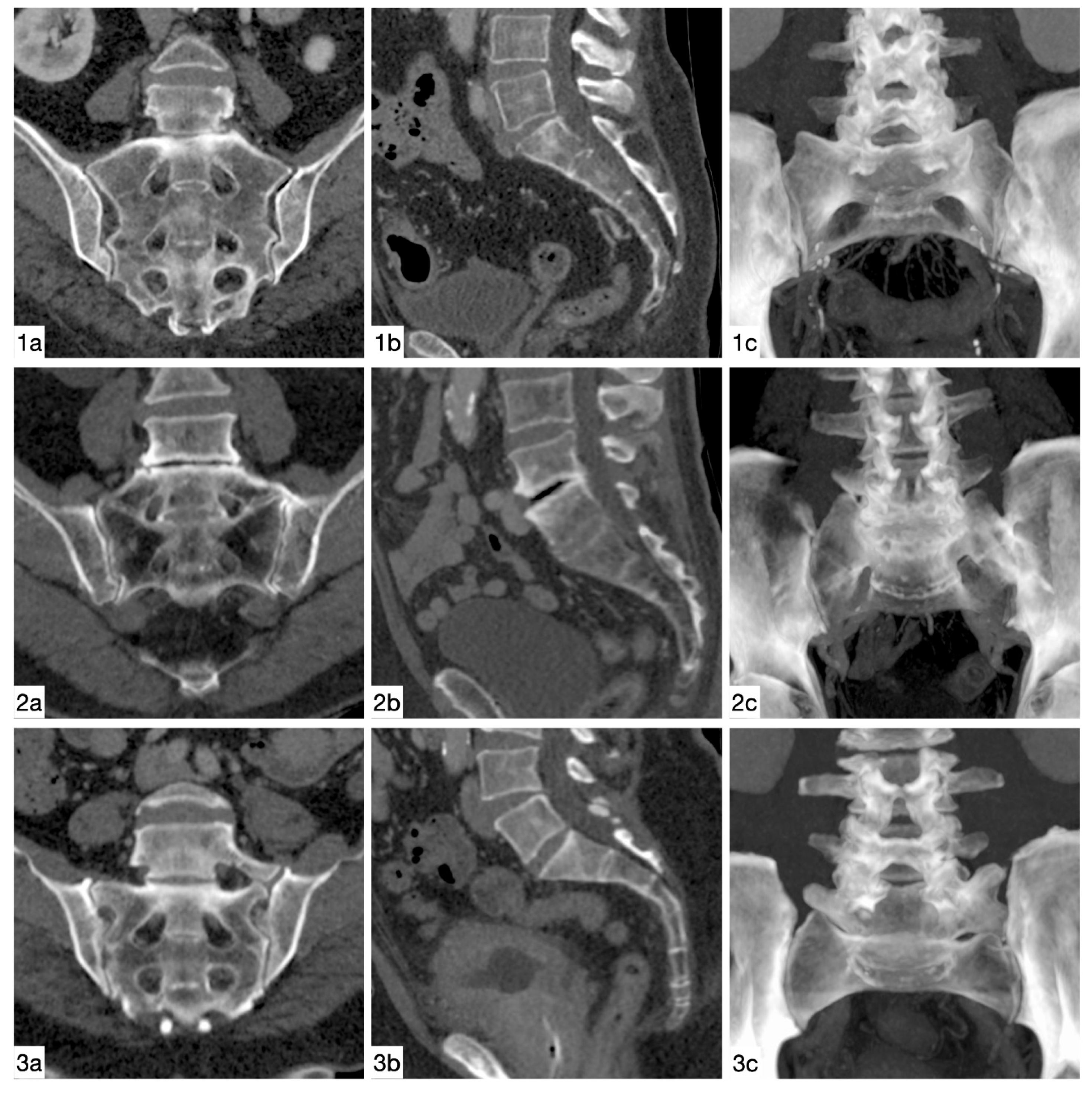
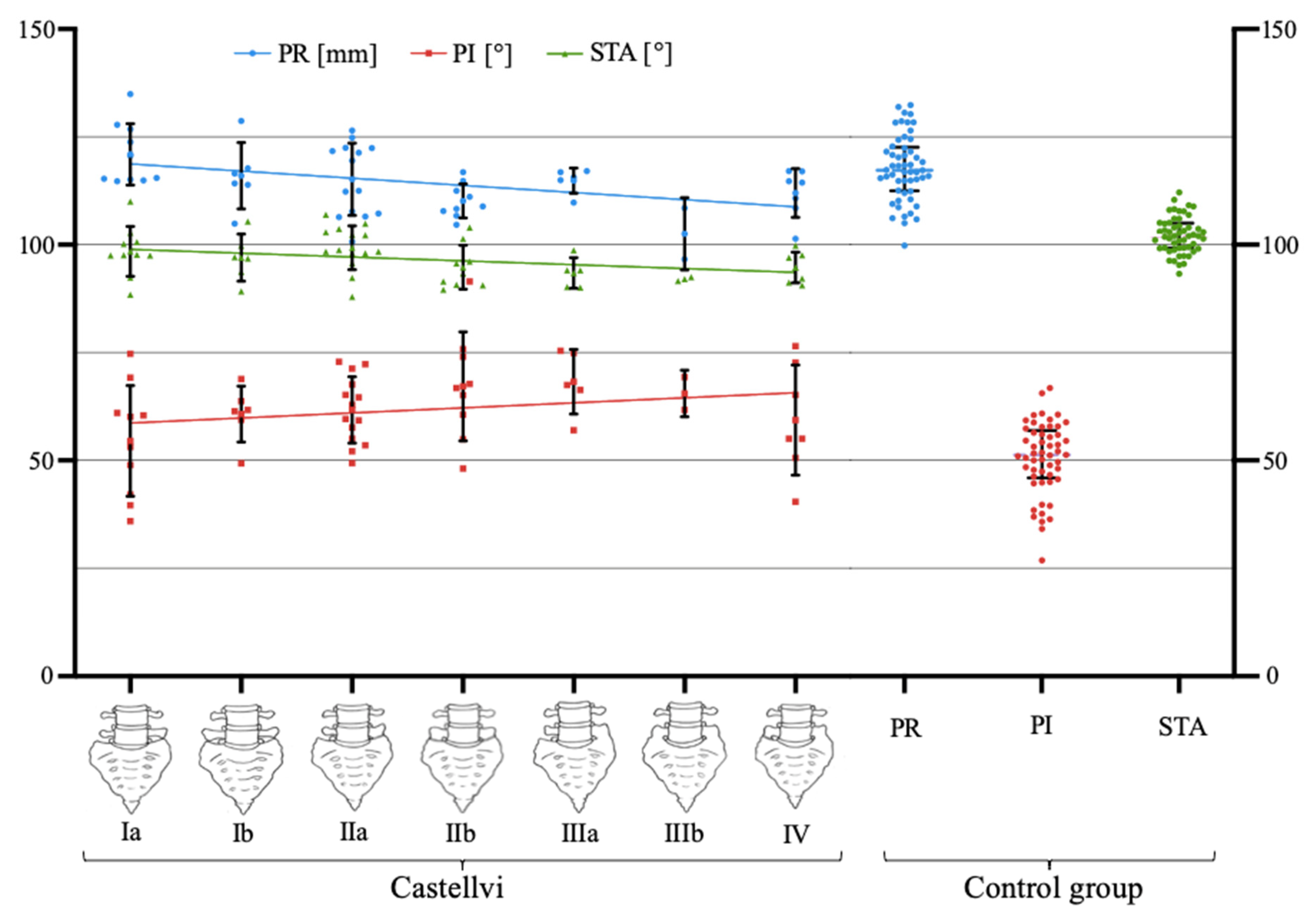
| Castellvi Type | Definition |
|---|---|
| Type I: dysplastic transverse process | Unilateral (A) or bilateral (B) dysplastic transverse process with a height >19 mm |
| Type II: incomplete lumbarization/sacralization | Enlarged transverse process with unilateral (A) or bilateral (B) pseudoarthrosis with the adjacent sacral ala |
| Type III: complete lumbarization/sacralization | Enlarged transverse process, which has a unilateral (A) or bilateral (B) complete fusion with the adjacent sacral ala |
| Type IV: mixed | Type II on one side and type III on the contralateral side |
| n | Mean | SD | Range | p-Value | |
|---|---|---|---|---|---|
| PR LSTV | 53 | 114.5 | 7.6 | 96.6–134.9 | 0.051 |
| PR Control | 53 | 117.8 | 7.6 | 99.8–132.4 | |
| PI LSTV | 53 | 61.6 | 10.8 | 35.8–91.5 | 0.001 * |
| PI Control | 53 | 50.5 | 8.4 | 26.8–66.8 | |
| STA LSTV | 53 | 96.7 | 5.2 | 87.9–110.0 | 0.001 * |
| STA Control | 53 | 102.2 | 4.2 | 93.3–121.1 |
| n | Mean | SD | Range | p-Value | |
|---|---|---|---|---|---|
| PR 6 LV S1 | 11 | 120.0 | 11.2 | 107.8–138.6 | 0.182 |
| PR 6 LV S2 | 11 | 114.2 | 4.5 | 107.8–122.5 | 0.859 |
| PR Control | 11 | 113.7 | 4.7 | 107.2–121.5 | |
| PI 6 LV S1 | 11 | 54.6 | 14.1 | 35.0–75.4 | 0.286 |
| PI 6 LV S2 | 11 | 65.3 | 8.5 | 48.1–73.4 | 0.010 * |
| PI Control | 11 | 48.5 | 10.2 | 26.8–57.9 | |
| STA 6 LV S1 | 11 | 97.2 | 4.2 | 89.2–109.4 | 0.010 * |
| STA 6 LV S2 | 11 | 95.1 | 4.2 | 89.2–101.4 | 0.004 * |
| STA Control | 11 | 103.0 | 4.0 | 99.1–110.1 |
| n | Mean | SD | Range | p-Value | |
|---|---|---|---|---|---|
| PR 4 LV | 9 | 111.3 | 7.6 | 96.6–117.1 | 0.678 |
| PR Control | 9 | 107.3 | 4.8 | 113.1–121.6 | |
| PI 4 LV | 9 | 62.5 | 8.8 | 50.6–75.4 | 0.021 * |
| PI Control | 9 | 52.8 | 6.5 | 38.5–57.9 | |
| STA 4 LV | 9 | 95.2 | 3.2 | 91.2–99.8 | 0.011 * |
| STA Control | 9 | 102.8 | 4.0 | 99.1–109.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haffer, H.; Becker, L.; Putzier, M.; Wiethölter, M.; Ziegeler, K.; Diekhoff, T.; Pumberger, M.; Hardt, S. Changes of Fixed Anatomical Spinopelvic Parameter in Patients with Lumbosacral Transitional Vertebrae: A Matched Pair Analysis. Diagnostics 2021, 11, 59. https://doi.org/10.3390/diagnostics11010059
Haffer H, Becker L, Putzier M, Wiethölter M, Ziegeler K, Diekhoff T, Pumberger M, Hardt S. Changes of Fixed Anatomical Spinopelvic Parameter in Patients with Lumbosacral Transitional Vertebrae: A Matched Pair Analysis. Diagnostics. 2021; 11(1):59. https://doi.org/10.3390/diagnostics11010059
Chicago/Turabian StyleHaffer, Henryk, Luis Becker, Michael Putzier, Mats Wiethölter, Katharina Ziegeler, Torsten Diekhoff, Matthias Pumberger, and Sebastian Hardt. 2021. "Changes of Fixed Anatomical Spinopelvic Parameter in Patients with Lumbosacral Transitional Vertebrae: A Matched Pair Analysis" Diagnostics 11, no. 1: 59. https://doi.org/10.3390/diagnostics11010059
APA StyleHaffer, H., Becker, L., Putzier, M., Wiethölter, M., Ziegeler, K., Diekhoff, T., Pumberger, M., & Hardt, S. (2021). Changes of Fixed Anatomical Spinopelvic Parameter in Patients with Lumbosacral Transitional Vertebrae: A Matched Pair Analysis. Diagnostics, 11(1), 59. https://doi.org/10.3390/diagnostics11010059







