Synovial Tissue Proteins and Patient-Specific Variables as Predictive Factors for Temporomandibular Joint Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
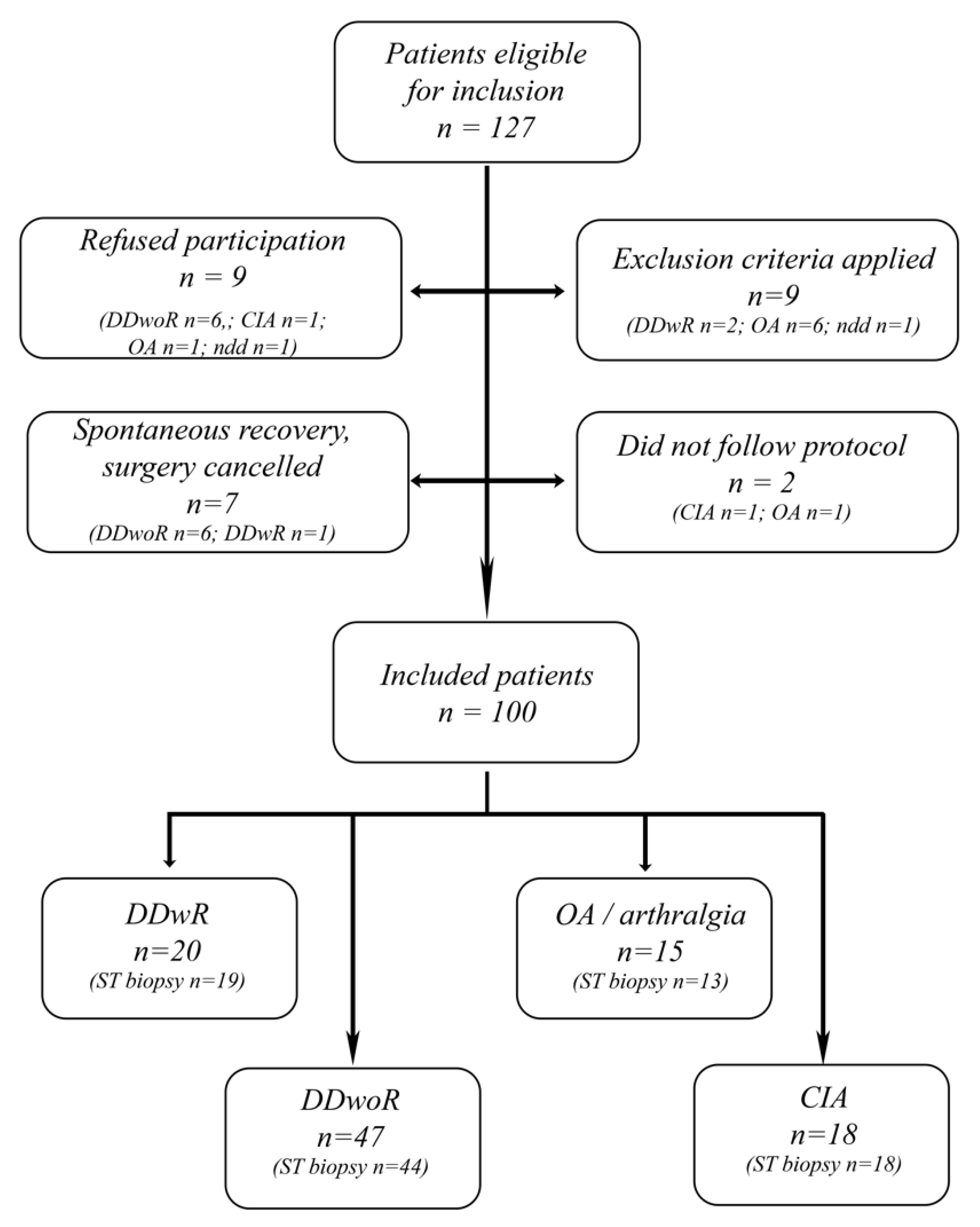
2.2. Study Population
2.3. Clinical Examination
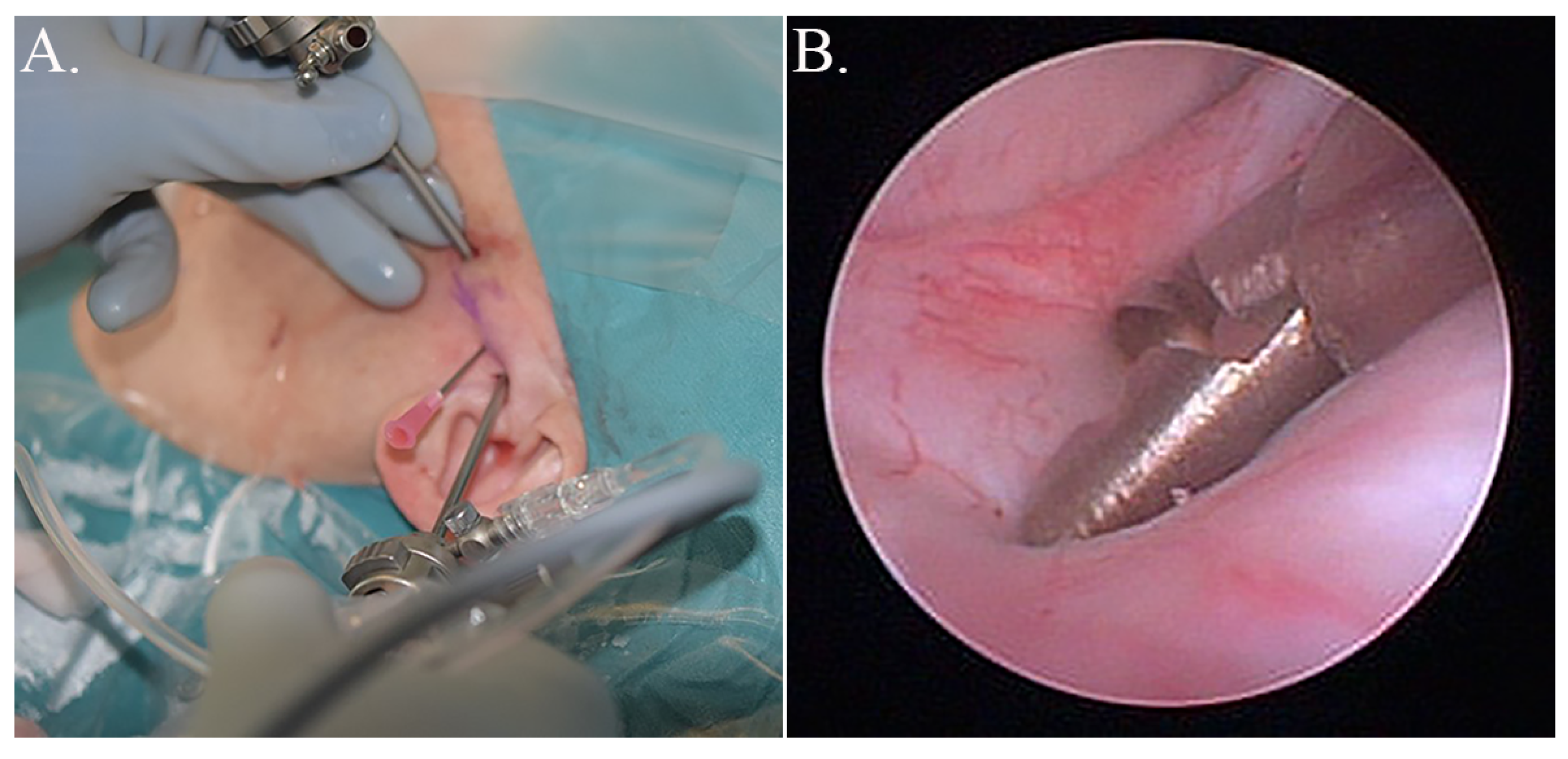
2.4. Surgical Procedure and Collection of Tissue Samples
2.5. Analysis of Synovial Tissues
2.6. Statistical Analyses
3. Results
3.1. Patient Demographics and Patient-Specific Clinical Variables
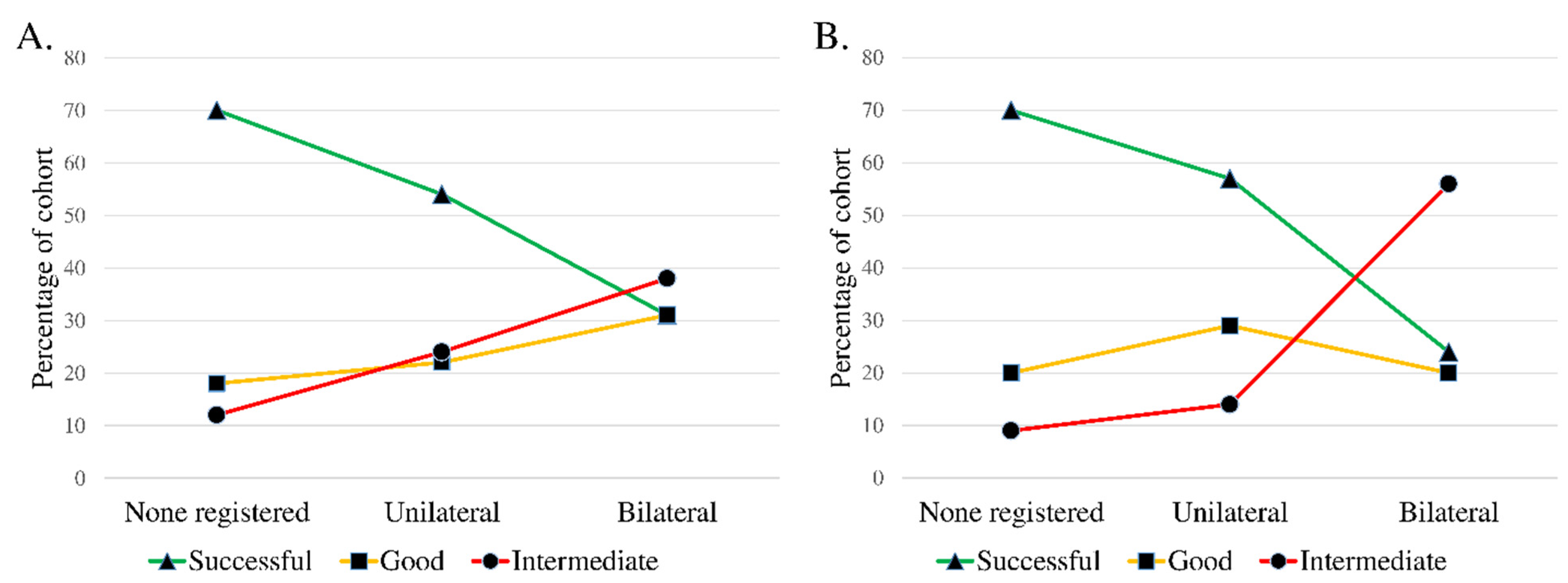
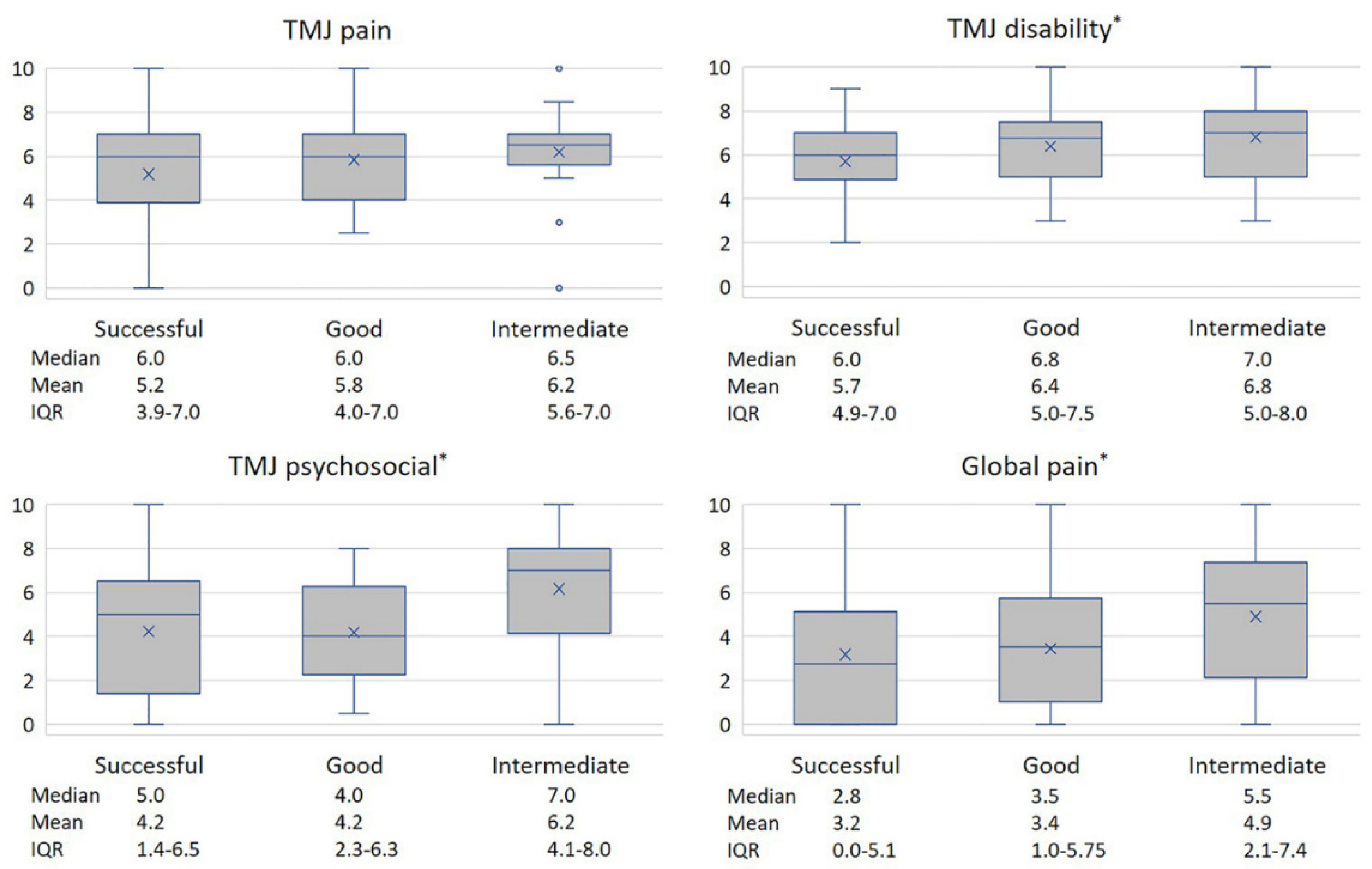
3.2. Synovial Tissue Analysis, Univariate Analysis
3.3. Multivariate Analysis of Synovial Tissue and Potential Confounders
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fillingim, R.B.; Slade, G.D.; Greenspan, J.D.; Dubner, R.; Maixner, W.; Bair, E.; Ohrbach, R. Long-term changes in biopsychosocial characteristics related to temporomandibular disorder: Findings from the OPPERA study. Pain 2018, 159, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Ulmner, M.; Weiner, C.K.; Lund, B. Predictive factors in temporomandibular joint arthroscopy: A prospective cohort short-term outcome study. Int. J. Oral Maxillofac. Surg. 2020, 49, 614–620. [Google Scholar] [CrossRef]
- Gynther, G.W.; Holmlund, A.B. Efficacy of arthroscopic lysis and lavage in patients with temporomandibular joint symptoms associated with generalized osteoarthritis or rheumatoid arthritis. J. Oral Maxillofac. Surg. 1998, 56, 147–151. [Google Scholar] [CrossRef]
- Machoň, V.; Šedý, J.; Klíma, K.; Hirjak, D.; Foltán, R. Arthroscopic lysis and lavage in patients with temporomandibular anterior disc displacement without reduction. Int. J. Oral Maxillofac. Surg. 2012, 41, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Miloro, M.; McKnight, M.; Han, M.D.; Markiewicz, M.R. Discectomy without replacement improves function in patients with internal derangement of the temporomandibular joint. J. Cranio-Maxillofacial Surg. 2017, 45, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, A.; Lund, B.; Weiner, C.K. Discectomy without replacement for the treatment of painful reciprocal clicking or catching and chronic closed lock of the temporomandibular joint: A clinical follow-up audit. Br. J. Oral Maxillofac. Surg. 2013, 51, 211–214. [Google Scholar] [CrossRef]
- Al-Moraissi, E. Open versus arthroscopic surgery for the management of internal derangement of the temporomandibular joint: A meta-analysis of the literature. Int. J. Oral Maxillofac. Surg. 2015, 44, 763–770. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the international RDC/TMD consortium network* and orofacial pain special interest groupdagger. J Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Lund, B.; Ulmner, M.; Bjørnland, T.; Berge, T.; Olsen-Bergem, H.; Rosèn, A. A disease-focused view on the temporomandibular joint using a Delphi-guided process. J. Oral Sci. 2020, 62, 1–8. [Google Scholar] [CrossRef]
- Haeffs, T.H.; D’Amato, L.N.; Khawaja, S.N.; Keith, D.A.; Scrivani, S.J. What variables are associated with the outcome of arthroscopic lysis and lavage surgery for internal derangement of the temporomandibular joint? J. Oral Maxillofac. Surg. 2018, 76, 2081–2088. [Google Scholar] [CrossRef]
- Bouloux, G.F.; Zerweck, A.G.; Celano, M.; Dai, T.; Easley, K.A. Can preoperative psychological assessment predict outcomes after temporomandibular joint arthroscopy? J. Oral Maxillofac. Surg. 2015, 73, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Israel, H. Does the age of a patient affect the outcome of temporomandibular joint arthroscopic surgery? J. Oral Maxillofac. Surg. 2017, 75, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Ulmner, M.; Kruger-Weiner, C.; Lund, B. Patient-specific factors predicting outcome of temporomandibular joint arthroscopy: A 6-year retrospective study. J. Oral Maxillofac. Surg. 2017, 75, 1643. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Kondoh, T.; Holmlund, A.B.; Sakota, K.; Nomura, Y.; Seto, K. Cytokine and clinical predictors for treatment outcome of visually guided temporomandibular joint irrigation in patients with chronic closed lock. J. Oral Maxillofac. Surg. 2008, 66, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Orr, C.; Vieira-Sousa, E.; Boyle, D.L.; Buch, M.H.; Buckley, C.D.; Cañete, J.D.; Catrina, A.I.; Choy, E.H.S.; Emery, P.; Fearon, U.; et al. Synovial tissue research: A state-of-the-art review. Nat. Rev. Rheumatol. 2017, 13, 463–475. [Google Scholar] [CrossRef]
- Veale, D.J. Synovial tissue biopsy research. Front. Med. 2019, 6, 72. [Google Scholar] [CrossRef]
- Holmlund, A. Disc derangements of the temporomandibular joint: A tissue-based characterization and implications for surgical treatment. Int. J. Oral Maxillofac. Surg. 2007, 36, 571–576. [Google Scholar] [CrossRef]
- Beighton, P.; Horan, F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J. Bone Jt. Surgery. Br. 1969, 51, 444–453. [Google Scholar] [CrossRef]
- Wilkes, C.H. Internal derangements of the temporomandibular joint: Pathological variations. Arch. Otolaryngol. Head Neck Surg. 1989, 115, 469–477. [Google Scholar] [CrossRef]
- McCain, J.P. Principles and Practice of Temporomandibular Joint Arthroscopy; Mosby: Maryland Heights, MO, USA, 1996. [Google Scholar]
- Rosengren, S.; Firestein, G.S.; Boyle, D.L. Measurement of inflammatory biomarkers in synovial tissue extracts by enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 2003, 10, 1002–1010. [Google Scholar] [CrossRef]
- Hamada, Y.; Kondoh, T.; Holmlund, A.B.; Yamamoto, M.; Horie, A.; Saito, T.; Ito, K.; Seto, K.; Sekiya, H. Inflammatory cytokines correlated with clinical outcome of temporomandibular joint irrigation in patients with chronic closed lock. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Ulmner, M.; Sugars, R.; Naimi-Akbar, A.; Suslu, S.; Reseland, J.E.; Kruger-Weiner, C.; Lund, B. Synovial tissue cytokine profile in disc displacement of the temporomandibular joint. J. Oral Rehabilitation 2020, 47, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Khurram, S.A.; Bingle, L.; McCabe, B.M.; Farthing, P.M.; Whawell, S.A. The chemokine receptors CXCR1 and CXCR2 regulate oral cancer cell behaviour. J. Oral Pathol. Med. 2014, 43, 667–674. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Moura, Á.F.B.; Barbosa, G.A.S.; Pereira, H.S.G.; Calderon, P.D.S. Do matrix metalloproteinases play a role in degenerative disease of temporomandibular joint? a systematic review. CRANIO 2016, 34, 112–117. [Google Scholar] [CrossRef]
- Itoh, Y. Metalloproteinases in rheumatoid arthritis: Potential therapeutic targets to improve current therapies. Prog. Mol. Biol. Transl. Sci. 2017, 148, 327–338. [Google Scholar] [CrossRef]
- Edman, K.; Furber, M.; Hemsley, P.; Johansson, C.; Pairaudeau, G.; Petersen, J.; Stocks, M.; Tervo, A.; Ward, A.; Wells, E.; et al. The discovery of MMP7 inhibitors exploiting a novel selectivity trigger. ChemMedChem 2011, 6, 769–773. [Google Scholar] [CrossRef]
- Liu, J.; Khalil, R.A. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog. Mol. Biol. Transl. Sci. 2017, 148, 355–420. [Google Scholar] [CrossRef]
- Zafarullah, M.; Su, S.; Martel-Pelletier, J.; Dibattista, J.A.; Costello, B.G.; Stetler-Stevenson, W.G.; Pelletier, J.-P. Tissue inhibitor of metalloproteinase-2 (TIMP-2) mRNA is constitutively expressed in bovine, human normal, and osteoarthritic articular chondrocytes. J. Cell. Biochem. 1996, 60, 211–217. [Google Scholar] [CrossRef]
- Ko, J.H.; Kang, Y.M.; Yang, J.H.; Kim, J.S.; Lee, W.J.; Kim, S.H.; Yang, I.H.; Moon, S.-H. Regulation of MMP and TIMP expression in synovial fibroblasts from knee osteoarthritis with flexion contracture using adenovirus-mediated relaxin gene therapy. Knee 2019, 26, 317–329. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Yu, W.-B.; Luo, W.; Gao, S.-G.; Li, Y.-S.; Lei, G.-H. Effect of osteopontin on TIMP-1 and TIMP-2 mRNA in chondrocytes of human knee osteoarthritis in vitro. Exp. Ther. Med. 2014, 8, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, K.; Perrot, G.; Maquart, F.-X.; Brézillon, S. Lumican as a multivalent effector in wound healing. Adv. Drug Deliv. Rev. 2018, 129, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Kiga, N.; Tojyo, I.; Matsumoto, T.; Hiraishi, Y.; Shinohara, Y.; Fujita, S. Expression of lumican in the articular disc of the human temporomandibular joint. Eur. J. Histochem.: EJH 2010, 54, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kindstedt, E.; Holm, C.K.; Sulniute, R.; Martinez-Carrasco, I.; Lundmark, R.; Lundberg, P. CCL11, a novel mediator of inflammatory bone resorption. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Pap, T.; Bertrand, J. Syndecans in cartilage breakdown and synovial inflammation. Nat. Rev. Rheumatol. 2012, 9, 43–55. [Google Scholar] [CrossRef]
- Bi, R.; Yin, Q.; Mei, J.; Chen, K.; Luo, X.; Fan, Y.; Zhu, S. Identification of human temporomandibular joint fibrocartilage stem cells with distinct chondrogenic capacity. Osteoarthr. Cartil. 2020, 28, 842–852. [Google Scholar] [CrossRef]
- Kurita, K.; Goss, A.N.; Ogi, N.; Toyama, M. Correlation between preoperative mouth opening and surgical outcome after arthroscopic lysis and lavage in patients with disc displacement without reduction. J. Oral Maxillofac. Surg. 1998, 56, 1394–1397. [Google Scholar] [CrossRef]
- Breik, O.; Devrukhkar, V.; Dimitroulis, G. Temporomandibular joint (TMJ) arthroscopic lysis and lavage: Outcomes and rate of progression to open surgery. J. Cranio-Maxillofacial Surg. 2016, 44, 1988–1995. [Google Scholar] [CrossRef]
| Classification | DDwR | DDwoR | OA | CIA | Total |
|---|---|---|---|---|---|
| Demographic data | |||||
| Number of patients | 20 | 47 | 15 | 18 | 100 |
| Sex, W/M | 15/5 | 40/7 | 15/0 | 17/1 | 87/13 |
| Age (years), mean (SD) | 37.5 (11.7) | 43.1 (15.8) | 50.7 (20.3) | 38.1 (13.0) | 42.2 (15.8) |
| Patient history | |||||
| Duration (mos.) mean (SD) | 43.3 (40.4) | 20.7 (24.0) | 29.3 (44.1) | 43.5 (42.7) | 30.4 (35.7) |
| Tinnitus/ear fullness, n (%) | 5 (25) | 14 (30) | 5 (33) | 5 (28) | 29 (29) |
| TMJ trauma, n (%) | 8 (40) | 10 (21) | 3 (20) | 3 (17) | 24 (24) |
| Medical history, n (%) | |||||
| Healthy | 10 (50) | 19 (40) | 2 (13) | 0 (0) | 31 (31) |
| Psychiatric disorder | 4 (20) | 15 (32) | 1 (7) | 3 (17) | 23 (23) |
| Neuropsychiatric disorder | 1 (5) | 1 (2) | 0 (0) | 1 (6) | 3 (3) |
| Autoimmune disease | 0 (0) | 0 (0) | 1 (7) | 18 (100) | 19 (19) |
| Metabolic disease | 2 (10) | 6 (13) | 3 (20) | 0 (0) | 11 (11) |
| Other disease | 6 (30) | 22 (47) | 11 (73) | 10 (56) | 49 (49) |
| Objective measures | |||||
| MIO, mm (SD) | 43.8 (9.7) | 29.2 (4.7) | 40.4 (5.7) | 31.2 (7.0) | 34.1 (8.9) |
| Wilks classification, mean (SD) | 2.6 (0.9) | 3.9 (0.6) | na | na | 3.6 (1.0) |
| TMJ palp pain, n (bilat/lat/no) | 0/6/14 | 4/28/15 | 2/10/3 | 7/10/1 | 13/54/33 |
| Muscle palp pain, n (bilat/lat/no) | 2/4/14 | 7/10/28 | 6/3/6 | 9/3/5 | 24/20/53 |
| Subjective measures (VAS 0-10), mean (SD) | |||||
| TMJ pain | 4.3 (2.5) | 5.6 (2.4) | 6.3 (2.2) | 6.2 (1.9) | 5.6 (2.4) |
| TMJ disability | 6.1 (2.0) | 6.3 (1.7) | 5.7 (2.2) | 5.8 (1.8) | 6.1 (1.8) |
| TMJ psychosocial impact | 5.1 (3.3) | 3.7 (2.8) | 5.7 (3.6) | 5.7 (2.4) | 4.6 (3.0) |
| Global pain | 3.1 (3.2) | 3.0 (3.0) | 4.9 (2.5) | 4.8 (2.9) | 3.6 (3.0) |
| Outcome | Successful | Good | Intermediate | Deteriorated a |
|---|---|---|---|---|
| Number of patients (%) | ||||
| Total | 56 (56) | 22 (22) | 21 (21) | 1 (1) |
| DDwR | 14 (70) | 3 (15) | 3 (15) | 0 (0) |
| DDwoR | 24 (51) | 14 (30) | 9 (19) | 0 (0) |
| OA | 9 (60) | 4 (27) | 2 (13) | 0 (0) |
| CIA | 9 (50) | 1 (6) | 7 (39) | 1 (6) |
| Preoperative measurements sorted according to outcome | ||||
| Sex, W/M | 48/8 | 18/4 | 20/1 | |
| Age (years), mean (SD) | 44.5 (16.0) | 39.1 (16.2) | 39.4 (14.6) | |
| Patient history | ||||
| Duration (mos.) mean (SD) | 30.9 (39.5) | 30.0 (31.1) | 30.5 (31.8) | |
| Tinnitus/ear fullness, n (%) | 13 (23) | 7 (32) | 9 (43) | |
| TMJ trauma, n (%) | 15 (27) | 7 (32) | 2 (10) | |
| GJH, n (%) | 12 (21) | 7 (32) | 5 (23) | |
| Medical history, n (%) | ||||
| Healthy | 19 (34) | 6 (27) | 6 (29) | |
| Psychiatric disorder | 12 (21) | 5 (23) | 6 (29) | |
| Neuropsychiatric disorder | 0 (0) | 2 (9) | 1 (5) | |
| Autoimmune disease | 1 (2) | 1 (5) | 1 (5) | |
| Metabolic disease | 7 (13) | 3 (14) | 1 (5) | |
| Other disease | 26 (46) | 13 (59) | 10 (48) | |
| Objective measures, mean (SD) | ||||
| MIO (mm) | 34.4 (7.6) | 33.9 (11.8) | 33.5 (9.3) | |
| Wilks classification | 3.5 (1.0) | 3.6 (0.9) | 4.0 (0.9) | |
| Mean differences of pre- and postoperative measurements in relation to outcome | ||||
| Objective measures (mm), mean (SD) | ||||
| MIO | 8.4 (7.2) ** | 7.1 (8.0) ** | 0.5 (7.2) | |
| LTR left | 0.3 (2.2) | 1.9 (2.8) * | -0.7 (3.0) | |
| LTR right | 0.8 (3.1) * | 0.3 (2.8) | 0.7 (2.1) | |
| PTR | 1.5 (2.9) ** | 1.7 (2.6) * | 0.6 (2.0) | |
| Subjective measures (VAS 0-10), mean (SD) | ||||
| TMJ pain | −4.1 (2.4) ** | −1.6 (2.4) ** | −0.7 (1.7) | |
| TMJ disability | −4.2 (1.8) ** | −2.5 (2.2) ** | −0.5 (1.9) | |
| TMJ psychosocial | −3.5 (2.6) ** | −0.8 (3.2) | −0.1 (2.6) | |
| Global pain | −1.2 (2.8) ** | −0.3 (2.0) | 0.5 (3.3) | |
| No. obs. | Specified Protein a | CIA b | Masticatorymuscle Palpation b | TMJ Disability b | Interaction CIA/Palp.c | |
|---|---|---|---|---|---|---|
| Protein | Coef. | Coef. | Coef. | Coef. | Coef. | |
| ADAMTS13 | 87 | 7.29 × 10−7 | 3.78 ** | 2.15 ** | 0.27 * | −4.19 * |
| Aggrecan | 94 | −8.25 × 10−6 | 1.77 | 1.93 ** | 0.22 | −2.07 |
| BMP-2 | 94 | −9.06 × 10−5 | 1.24 | 1.87 ** | 0.23 | −1.69 |
| BMP-4 | 94 | 8.05 × 10−4 | 1.34 | 1.95 ** | 0.22 | −1.62 |
| BMP-9 | 44 | 1.45 × 10−3 | 1.00 | 1.73 | 0.24 | −1.54 |
| Collagen-1 α1 | 94 | 4.28 × 10−6 | 1.31 | 1.90 ** | 0.21 | −1.66 |
| Collagen-4 α1 | 94 | −2.38 × 10−6 | 1.15 | 1.86 ** | 0.23 | −1.64 |
| EGF | 94 | 4.33 × 10−4 | 1.16 | 1.86 ** | 0.22 | −1.64 |
| Eotaxin | 93 | 2.69 × 10−3 | 1.19 | 1.92 ** | 0.22 | −1.61 |
| FAP | 94 | 1.05 × 10−5 | 1.37 | 1.95 ** | 0.18 | −1.72 |
| FGF-2 | 94 | 4.03 × 10−5 | 1.22 | 1.99 ** | 0.23 | −1.77 |
| Fibronectin | 94 | 5.12 × 10−8 | 1.26 | 1.83 * | 0.20 | −1.66 |
| G-CSF | 94 | −2.32 × 10−4 | 1.82 | 2.01 ** | 0.22 | −2.37 |
| HGFR | 94 | 6.68 × 10−5 | 1.34 | 1.92 ** | 0.21 | −1.68 |
| ICAM-1 | 94 | 1.54 × 10−8 | 1.17 | 1.86 ** | 0.22 | −1.65 |
| IL-1β | 85 | −2.57 × 10−2 | 1.98 * | 2.01 ** | 0.20 | −3.27 * |
| IL-1ra | 94 | 2.51 × 10−3 | 1.24 | 1.90 ** | 0.21 | −1.64 |
| IL-6 | 46 | 8.66 × 10−3 | 1.33 | 0.91 | 0.04 | −0.55 |
| IL-7 | 94 | −6.60 × 10−4 | 1.54 | 1.91 ** | 0.22 | −2.01 |
| IL-8 | 93 | 2.17 × 10−2 * | 1.11 | 2.11 ** | 0.20 | −1.33 |
| IL-10 | 92 | 5.66 × 10−3 | 1.56 | 1.88 * | 0.25 * | −2.00 |
| IP-10 | 94 | 6.99 × 10−5 | 1.13 | 1.68 * | 0.23 | −1.40 |
| Lumican | 94 | 9.99 × 10−8 * | 1.48 | 2.02 ** | 0.17 | −1.82 |
| MCP-1 | 94 | −2.43 × 10−4 | 1.09 | 1.91 ** | 0.23 | −1.53 |
| MIP-1α | 49 | 1.70 × 10−3 | 1.71 | 1.97 | −0.09 | −2.80 |
| MIP-1β | 60 | 3.61 × 10−3 | 1.73 * | 1.63 * | 0.16 | −1.70 |
| MMP-1 | 66 | 9.72 × 10−4 | 17.74 | 2.56 ** | 0.26 | − d |
| MMP-2 | 93 | 1.92 × 10−7 | 1.55 | 1.86 ** | 0.24 | −2.04 |
| MMP-7 | 62 | 3.06 × 10−5 * | − e | 3.22 ** | 0.23 | − e |
| MMP-9 | 93 | 1.92 × 10−6 | 1.61 * | 1.90 ** | 0.24 | −2.07 |
| MMP-10 | 89 | −5.65 × 10−5 | 1.57 | 1.80 * | 0.26 * | −2.02 |
| NCAM-1 | 94 | 1.74 × 10−5 | 1.33 | 1.90 ** | 0.22 | −1.58 |
| OPG | 94 | 1.73 × 10−5 | 1.31 | 1.80 * | 0.20 | −1.61 |
| Osteonectin | 94 | 2.98 × 10−8 | 1.17 | 1.86 ** | 0.22 | −1.66 |
| PDGF-AA | 94 | 1.28 × 10−4 | 1.16 | 1.84 * | 0.22 | −1.61 |
| PDGF-AB/BB | 94 | −4.84 × 10−5 | 1.27 | 1.88 ** | 0.21 | −1.84 |
| RANTES | 94 | −4.13 × 10−5 | 1.22 | 1.80 * | 0.22 | −1.92 |
| Syndecan-1 | 94 | 7.62 × 10−5 | 0.60 | 1.72 * | 0.19 | −0.98 |
| Syndecan-4 | 90 | 9.74 × 10−5 | 1.20 | 1.89 ** | 0.19 | −1.66 |
| Tenascin C | 94 | 4.13 × 10−6 | 1.23 | 1.89 ** | 0.20 | −1.70 |
| TIMP-1 | 93 | 3.91 × 10−5 | 2.01 * | 1.89 ** | 0.20 | −2.04 |
| TIMP-2 | 93 | 3.11 × 10−5 * | 2.12 * | 2.18 ** | 0.21 | −2.25 |
| TIMP-3 | 93 | 5.68 × 10−5 | 1.87 * | 2.02 ** | 0.25 * | −2.22 |
| TIMP-4 | 93 | 4.30 × 10−4 | 1.57 | 1.86 ** | 0.23 | −2.08 |
| TNF-α | 91 | −2.89 × 10−2 | 2.57 * | 1.91 ** | 0.27 * | −2.47 |
| TNF-β | 65 | −1.53 × 10−2 | 3.36 * | 3.03 ** | 0.11 | −4.48 * |
| TREM-1 | 85 | 9.48 × 10−5 | 1.18 | 1.60 * | 0.22 | −1.39 |
| VEGF | 93 | 6.03 × 10−4 | 1.49 | 1.79 * | 0.23 | −1.94 |
| GM-CSF f | 41 | − | − | − | − | − |
| IL-17 f | 13 | − | − | − | − | − |
| TGF-α f | 32 | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulmner, M.; Sugars, R.; Naimi-Akbar, A.; Tudzarovski, N.; Kruger-Weiner, C.; Lund, B. Synovial Tissue Proteins and Patient-Specific Variables as Predictive Factors for Temporomandibular Joint Surgery. Diagnostics 2021, 11, 46. https://doi.org/10.3390/diagnostics11010046
Ulmner M, Sugars R, Naimi-Akbar A, Tudzarovski N, Kruger-Weiner C, Lund B. Synovial Tissue Proteins and Patient-Specific Variables as Predictive Factors for Temporomandibular Joint Surgery. Diagnostics. 2021; 11(1):46. https://doi.org/10.3390/diagnostics11010046
Chicago/Turabian StyleUlmner, Mattias, Rachael Sugars, Aron Naimi-Akbar, Nikolce Tudzarovski, Carina Kruger-Weiner, and Bodil Lund. 2021. "Synovial Tissue Proteins and Patient-Specific Variables as Predictive Factors for Temporomandibular Joint Surgery" Diagnostics 11, no. 1: 46. https://doi.org/10.3390/diagnostics11010046
APA StyleUlmner, M., Sugars, R., Naimi-Akbar, A., Tudzarovski, N., Kruger-Weiner, C., & Lund, B. (2021). Synovial Tissue Proteins and Patient-Specific Variables as Predictive Factors for Temporomandibular Joint Surgery. Diagnostics, 11(1), 46. https://doi.org/10.3390/diagnostics11010046





