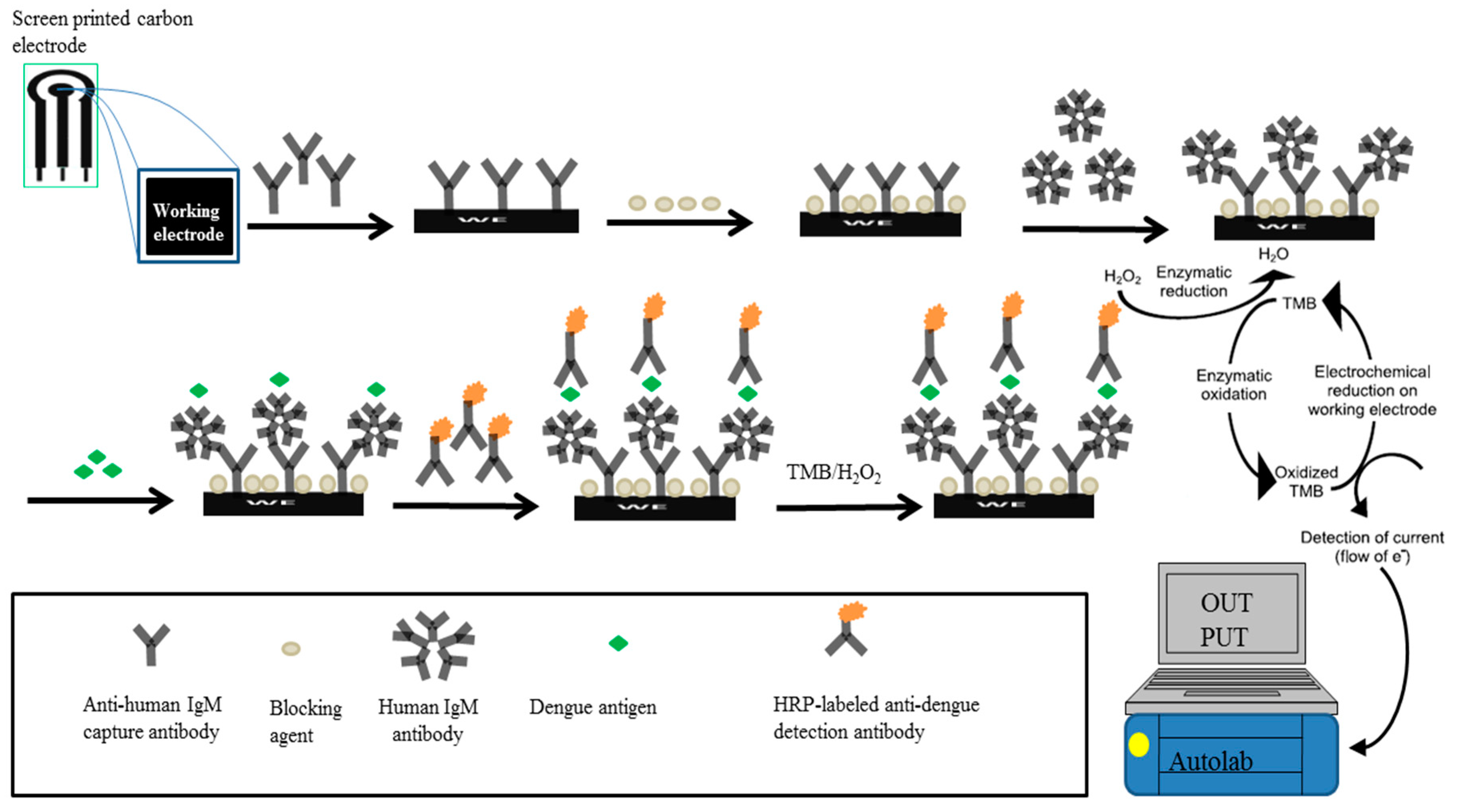
1. Introduction
Dengue is among the most important human viral diseases that exert a considerable global health burden. In the past few decades, the global prevalence of dengue has risen significantly. Currently, dengue, which poses risk to approximately 3.6 billion people, is endemic in over 100 tropical and subtropical countries. Annually, 390 million dengue cases are estimated to occur. Dengue patients often experience a variety of clinical manifestations, ranging from mild dengue fever to life-threatening severe dengue [
1,
2]. Although a vaccine is now commercially available, it is not widely accessible in many countries [
3]. Furthermore, clinical diagnosis could be challenging, since certain signs and symptoms of dengue overlap with other diseases, such as chikungunya, malaria, typhoid, typhus and leptospirosis [
4,
5]. Thus, effective management of dengue patients and subsequent outbreak control depend partly on rapid and accurate diagnosis of suspected patients [
6,
7].
Serological assays to detect the dengue-specific non-structural 1 (NS1) antigen or immunoglobulin M (IgM) antibodies are among the most common diagnostic methods used for the detection of dengue virus infection [
8,
9]. The most widely used serological assays are the lateral flow test and the enzyme-linked immunosorbent assay (ELISA) [
10]. The former test is rapid, taking between 10 to 30 min to complete, but tends to exhibit lower sensitivity and specificity than ELISA [
11]. In contrast, ELISA requires expensive equipment for its analysis and automation [
12].
Electrochemical biosensors are an emerging technology that could be incorporated into clinical diagnostic kits to enhance diagnosis, since it is highly sensitive, specific, simple, rapid and reliable [
13]. Compatibility with new microelectronic tools, minimum energy consumption, cost effectiveness and exclusion from sample turbidity and colour are other advantages of electrochemical biosensors [
14,
15]. An electrochemical biosensor employing screen printed carbon electrodes (SPCEs) is an attractive alternative to the current diagnostic method, as it demonstrates a sensitivity that is as good as ELISA and a portability comparable to the lateral flow assay. The strong chemical inertness of carbon electrodes provides a variety of working potential with low electrical resistivity. They also have a crystalline structure that provides low residual currents and a high signal to noise ratio [
16,
17]. In addition, the SPCEs electrochemical biosensor has advantages such as low production cost, simplicity, portability, versatility and ease of mass production.
We previously developed an electrochemical biosensor for the detection of dengue-specific non-structural 1 (NS1) antigen. The biosensor, which was designed based on the principle of direct ELISA and fabricated using SPCEs, exhibited good sensitivity and specificity [
18]. However, a more common approach for serological diagnosis of dengue in an endemic country, such as Malaysia, is the simultaneous detection of the dengue NS1 antigen and IgM antibodies. In infected patients, the dengue NS1 biomarker is detectable on the first day of fever. Several days following infection, the dengue NS1 level declines, while dengue-specific IgM and IgG antibodies start to elevate [
19]. Thus, concurrent detection of the dengue NS1 antigen and IgM antibodies increases the accuracy and ease of diagnosing dengue at any time-point of infection, averts the drawback of repeated testing and ultimately provides prompt diagnosis to doctors. This study therefore aimed to develop a sensitive electrochemical biosensor for detecting dengue IgM antibodies, with the expectation that, in the future, it could be combined with an NS1 biosensor as a complete test package for dengue. In this proposed IgM electrochemical biosensor, we used an approach similar to our previously developed NS1 biosensor. However, rather than the principle of direct ELISA utilized for the NS1 biosensor, this IgM electrochemical biosensor was constructed based on IgM capture ELISA (MAC-ELISA) [
18]. Consequently, the fabrication involved entirely different optimisation procedures and reagents than the previously developed NS1 biosensor. Furthermore, we demonstrated the potential application of the IgM biosensor on clinical samples.
2. Materials and Methods
2.1. SPCE, Chemicals and Assay Reagents
The SPCEs were bought from Scrint Technology (Penang, Malaysia). The SPCEs were based on three carbon electrodes, namely, the working electrode, counter electrode and reference electrode. The working electrode was used for developing an immunoassay, while the two other electrodes surrounded the working electrode to form a complete electrical circuit. The electrochemical characterisation, reproducibility and stability of the SPCEs have been evaluated in our previous study [
18]. Bovine serum albumin (BSA), ethanolamine hydrochloride, streptavidin and GAHICA were purchased from Sigma (St. Louis, MO, USA). BupH MES buffered saline packs were purchased from Thermo Scientific (Rockford, IL, USA). Lightning-link horseradish peroxidase (HRP) and lightning-link biotin were purchased from Innova Biosciences (Cambridge, UK). A recombinant polyvalent dengue antigen was purchased from RayBiotech (Norcross, GA, USA). Anti-dengue virus antibodies were acquired from Abcam (Cambridge, UK). Carboxymethyldextran (CMD) (500,000 MW) was purchased from Fluka (Gillingham, UK). Ready-to-use 3,30,5,50-tetramethylbenzidine (TMB) substrates were purchased from Promega (Madison, WI, USA) and the dengue IgM capture ELISA kit was purchased from Panbio Diagnostics (Brisbane, Australia).
2.2. Apparatus
Chronoamperometric measurements were performed with an Autolab PGSTAT III potentiostat/galvanostat (Metrohm Autolab B.V., Utrecht, The Netherlands), which was interfaced to Nova software (version 1.6, Metrohm Autolab B.V., Utrecht, The Netherlands).
2.3. Clinical Samples
Blood samples were obtained from suspected dengue patients visiting the clinic or emergency department at Hospital Universiti Sains Malaysia. The collected samples were tested with the dengue IgM capture ELISA (Panbio) at the Department of Medical Microbiology and Parasitology, Universiti Sains Malaysia (USM). Afterwards, ELISA positive and negative samples were pooled separately and used to develop and optimise the electrochemical biosensor. For diagnostic evaluation, 144 reference samples were provided by the Department of Microbiology, Universiti Malaya (UM) (
Table 1). This research has been approved by the USM Committee on Human Ethics (USM/JePeM/270.4.(1.3), 09/10/2013).
2.4. Immobilisation of GAHICA on SPCEs
A number of methods were evaluated to attain the best immobilisation strategy of GAHICA on the carbon working electrode (CWE). These strategies were either based on random antibody immobilisation (passive adsorption or covalent binding) or oriented antibody immobilisation (employing protein A, protein G or streptavidin-biotin).
2.4.1. Passive Adsorption
For passive adsorption, 20 µL (10 µg/mL) of GAHICA diluted in carbonate buffer (pH = 9.4) was pipetted on CWE. The SPCEs were then incubated overnight at 4 °C. Following washing, the CWE was blocked with 3% BSA for 15 min.
2.4.2. Covalent Immobilization
EDC/NHS, a chemical cross-linker, was used to immobilise GAHICA permanently on CWE of SPCEs. Initially, 20 µL (50 mg/mL) of CMD was added on the CWE to introduce the carboxylic group (COOH). Following overnight incubation, unbound CMD was washed away with PBS. Afterwards, the COOH group was activated on the CWE by incubating 20 µL of the cross-linkers (0.4 M of EDC and 0.1 M of NHS) for 10 min at room temperature. The cross-linker reacts with the COOH group and converts it into an active O-acylisourea intermediate, which is readily converted by nucleophilic attack from the primary amino group into an amide bond and isourea as a by-product. The amino group with primary reactive amine side chains on the capture antibodies was then linked by adding 20 µL (10 µg/mL) of GAHICA and incubated for 1 h. 20 µL of 1 M of ethanolamine hydrochloride was subsequently pipetted on the SPCEs for 10 min in the dark to remove unbound ester groups. Later, 3% BSA was added on the CWE for 15 min to block non-specific binding.
2.4.3. Protein A or G Based Immobilization
Protein A or G was immobilised on separate CWE by pipetting 20 µL (5 mg/mL) of each protein and incubating for 24 h. Subsequently, 20 μL (10 μg/mL) of GAHICA was incubated on the CWE for 1 h. Following washing, 20 μL of (3%) BSA was added on CWE for 15 min to block non-specific bindings.
2.4.4. Streptavidin-Biotin Based Immobilization
For a stable immobilization of streptavidin on the CWE, the covalent technique as stated in
Section 2.4.2 was used. Following incubation of EDC-NHS, 20 µL (50 µg/mL) of streptavidin was pipetted and incubated for 1 h. Upon washing, 20 µL/mL of biotinylated GAHICA (10 µg/mL) was pipetted on the CWE and incubated for 1 h. Then, 20 µL of 1 M of ethanolamine hydrochloride was pipetted on the CWE and incubated for 10 min in the dark. Afterwards, 20 µL of 3% BSA was added on the CWE for 15 min.
2.5. Visualisation of Immobilised GAHICA Using Field Emission Scanning Electron Microscopy (FESEM)
Following antibody immobilisation as described in
Section 2.4.4, the scanning electron micrograph images were obtained from FESEM at an acceleration voltage of 10 kV and a working distance of 10 μm.
2.6. Fabrication of Electrochemical Biosensor
Following successful immobilisation of GAHICA on the SPCEs, dengue IgM ELISA positive serum samples were pooled and used as a positive control/test, while pooled negative serum samples were used as a negative control. Pooled serum samples were diluted 1:100 in 0.01 M of PBS and then pipetted on the CWE to allow reaction with GAHICA for 1 h. Following incubation and washing, 20 µL (5 µg/mL) of dengue antigen prepared in PBS was added on the CWE. To complete the assay, 20 µL (5 µg/mL) of HRP-conjugated anti-human IgM detection antibodies was added on the CWE and left for 1 h. Before electrochemical measurement, the electrodes were washed with PBS. The cyclic voltammograms analysis following the immobilisation of GAHICA and other assay reagents showed a decrease in redox peaks, similar to that described in our previous study [
18], and therefore were not discussed in this study.
2.7. Quantification of Electrochemical Signals
To generate an electrochemical response, 70 µL of TMB substrate was pipetted on the three electrodes present on the SPCEs to immerse all three electrodes. Subsequently, electrochemical signals generated from the catalysis of H2O2 by the HRP enzyme were measured by applying a fixed reduction potential of −200 mV to the reference electrode. A cut-off value was established during each experiment to differentiate positive from negative results, and was obtained by calculating the average of three negative samples plus three times the standard deviation (SD).
2.8. Analytical and Diagnostic Evaluation of Dengue IgM Biosensor
For successful commercialisation, it is imperative that the established biosensor satisfies the criteria of a standard diagnostic test, such as exhibiting high sensitivity and specificity. Considering this fact, analytical and diagnostic evaluation was carried out for the developed biosensor.
2.8.1. Determination of Analytical Sensitivity
To determine the analytical sensitivity of the proposed biosensor, the pooled serum sample containing dengue-specific IgM antibodies was diluted from 10
1–10
7. These dilutions were later tested on the developed dengue IgM biosensor and a commercially available dengue IgM capture ELISA (Panbio). The biosensor assay was performed as mentioned in
Section 2.5, while the ELISA assay was carried out according to the protocol provided by the manufacturer.
2.8.2. Determination of Analytical Specificity
To ensure that the developed dengue IgM biosensor selectively detected dengue IgM antibodies, it was exposed to a panel of different samples containing IgM antibodies specific either for hepatitis B, hepatitis C, chikungunya, leptospirosis, malaria or healthy serum samples. The serum was diluted in 1:100 using PBS buffer.
2.8.3. Determination of Diagnostic Sensitivity and Specificity
Reference samples (
Table 1) were tested to assess the diagnostic sensitivity and specificity of the developed dengue IgM biosensor.
2.9. Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software, version 25. All of the assays were carried out on three independent SPCEs, and the values were presented as mean ± standard error (SE). One-way ANOVA with Tukey’s post hoc test was used for the analysis. A p-value ˂ 0.05 was considered statistically significant. Diagnostic sensitivity, specificity, PPV and NPV were calculated using the online MEDCALC® calculator.
4. Discussion
In this study, a highly specific dengue IgM biosensor was constructed and subsequently assessed for its future potential as an alternative diagnostic assay to confirm dengue infection. For the IgM-based biosensor, proper immobilisation of GAHICA on the CWE of SPCEs is a crucial step to ensure that a sensitive test is developed. This is because the sensitivity of the developed biosensor partly depends on the ability of the GAHICA to successfully bind the CWE with proper orientation of the antibodies. Among the techniques tested, covalent, protein A and protein G techniques generated slightly lower current signals. The lower current response observed during covalent based antibody immobilisation may be due to the effect of antibody crosslinking. The rather harsh treatment of cross-linkers and goat anti-human IgM antibodies could affect the biological activity of the antibodies [
20]. Similar to the finding in our previously developed dengue NS1 biosensor [
18], the low current signal obtained from the protein A and G immobilisation strategies could be due to the proteins’ weak/low binding affinities towards human IgM and IgA antibodies [
21]. In contrast, biotin is known to have strong affinities (ranging from 2.1 × 10
−3 to 1.7 × 10
−4 mol/L) toward IgM antibodies [
22]. Thus, with the aid of biotin, capture antibodies can be immobilised strongly and with good orientation on the carbon surface. Optimising the various assay reagents used in a biosensor assay is also pivotal to achieving the desired outcomes. Randomly selected concentrations may cause steric hindrance due to an inappropriate ratio of antigen and antibody, and could affect electrochemical responses. Hence, coupled with the use of optimised assay reagents, the performance of the developed IgM biosensor was generally superior to that of the conventional ELISA method.
High sensitivity is always a mandatory criterion for good diagnostic kits. This helps to address, particularly, the concern of a low concentration of antigen/antibodies in some clinical specimens. In terms of analytical sensitivity, the biosensor developed in this study surpassed the commercial dengue ELISA assay. In the future, this notable sensitivity can be further enhanced, perhaps by using a highly efficient electrochemical conductor, such as a gold electrode. Wong et al., 2014 and Atias et al., 2009 used an optical transduction approach for detecting dengue IgM antibodies [
2,
8]. The approach showed an analytical sensitivity similar to that found in this study. However, compared to optical transduction, a biosensor that employs electrochemical transduction can be easily developed as a point of care (POC) test when combined with a portable reader and integrated with a smartphone to deliver the results. Thus, the developed dengue IgM biosensor provides an appealing alternative POC test that incorporates the sensitivity and specificity of the ELISA assay and the portability of a rapid test.
Specificity is another critical component of a diagnostic assay. Cross-reactivity, a phenomenon that involves the interaction of specific antibodies with more than one antigen/agent, often leads to false positive outcomes in immunoassays, and could affect patient management. Thus, the specificity of an immunoassay typically lies in the quality of the antibodies used for the assay [
23]. In this study, the electrochemical biosensor selectively detected dengue-specific IgM antibody positive samples from other nonspecific pathogens. This high specificity of the IgM biosensor is attributable to the selective nature of the antibodies used, as well as the ELISA technique adopted for the biosensor. Similar levels of sensitivity and specificity demonstrated during the diagnostic evaluation of the biosensor suggest its future potential application in the diagnostic laboratory for the diagnosis of dengue in patients.
The IgM antibody is detectable in only 50% of patients from day 3–4 after the onset of illness, although this detection rate increases to 80% by day five and 99% by day 10 onwards [
24]. Therefore, serological diagnosis of the dengue IgM antibody is a less reliable test for acute dengue infection. Thus, to increase the detection rate of dengue, irrespective of the disease stage, the developed IgM biosensor should be combined with our previously developed NS1 biosensor [
16] for a concurrent detection of both biomarkers.
Notably, some of the reference samples collected on day five, six, seven and eight after the onset of illness were positive for the NS1 antigen and negative for the dengue IgM antibody. Interestingly, the electrochemical biosensor accurately recognized these samples as dengue IgM antibody negative samples. Theoretically, antibody titre is detectable from day five after the onset of fever. In this case, it is possible that these samples were not stored at the optimal temperature, thus, the structure of IgM antibodies may have been compromised. It is also possible that the samples might contain inhibitors that interfere with the antibody/antigen complex.
The developed dengue IgM biosensor demonstrated good analytical sensitivity and specificity, as well as diagnostic sensitivity and specificity. It is therefore a promising tool for the diagnosis of dengue infection after seroconversion. However, the diagnostic evaluation in this study was carried out using a limited number of reference samples. More reference samples, particularly from other closely related flaviviruses, including Japanese encephalitis virus and Zika virus, should be included in the future for a more robust validation of the dengue IgM biosensor.
5. Conclusions
In conclusion, an electrochemical biosensor based on the MAC-ELISA principle was successfully developed on screen printed carbon electrodes for the detection of dengue IgM antibodies. The developed biosensor demonstrated a high specificity as well as a high sensitivity with low LOD compared to commercial ELISA. Furthermore, diagnostic evaluation showed that the biosensor could be used for the detection of dengue IgM antibodies in real clinical serum samples. Therefore, the developed electrochemical biosensor provides a good alternative for rapid, sensitive and specific detection of dengue IgM antibodies in real serum samples. However, to decentralize the assay, the autolab reader utilised could be replaced in the future with a portable electrochemical reader or smartphone. Additionally, re-designing the developed biosensor as a multiplex assay with reduced operation time to diagnose different infections could enhance the usability, efficiency, and cost effectiveness of the biosensor in the future.