Health-Related Quality of Life 10 Years after Liver Transplantation: A Longitudinal Retrospective Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics
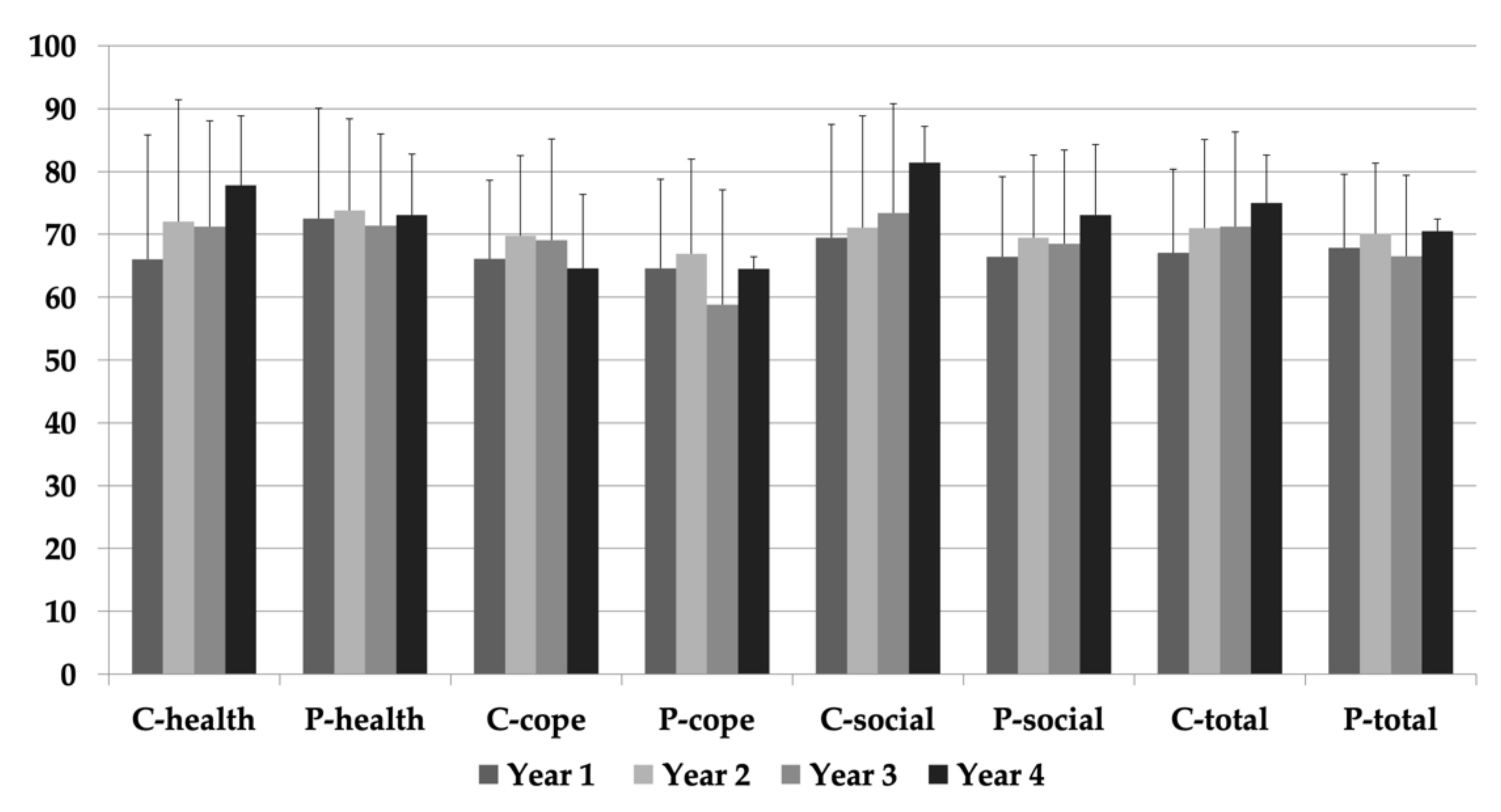
3.2. Health-Related Quality of Life
3.3. Ethnicity and Age
3.4. Medical Factors and Co-Morbidities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, L.T.; Carullo, P.C.; Banh, D.P.T.; Vitu, C.S.; Davis, P.J. Pediatric Liver Transplantation: Then and Now. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2028–2035. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Alonso, E.M.; Limbers, C.A.; Neighbors, K.; Martz, K.; Bucuvalas, J.C.; Webb, T.; Varni, J.W. Studies of Pediatric Liver Transplantation (SPLIT) Functional Outcomes Group (FOG) Cross-Sectional Analysis of Health-Related Quality of Life in Pediatric Liver Transplant Recipients. J. Pediatr. 2010, 156, 270–276.e1. [Google Scholar] [CrossRef] [PubMed]
- Bucuvalas, J.; Britto, M.; Krug, S.; Ryckman, F.C.; Atherton, H.; Alonso, M.P.; Balistreri, W.F.; Kotagal, U. Health-related quality of life in pediatric liver transplant recipients: A single-center study. Liver Transplant. 2003, 9, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, E.M.; Magee, J.C.; Shieck, V.; Well, A.; Lopez, M.J.; Opipari-Arrigan, L. Adherence and health-related quality of life in adolescent liver transplant recipients. Pediatr. Transplant. 2008, 12, 289–299. [Google Scholar] [CrossRef] [PubMed]
- LaRosa, C.; Baluarte, H.J.; Meyers, K.E. Outcomes in pediatric solid-organ transplantation. Pediatr. Transplant. 2011, 15, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Miserachs, M.; Nicholas, D.B.; Otley, A.R.; Ng, V.L. Health-Related Quality of Life after Pediatric Liver Transplantation: A Qualitative Analysis of the Perspectives of Health Care Providers. Can. J. Gastroenterol. Hepatol. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Windsorová, D.; Stewart, S.M.; Lovitt, R.; Waller, D.A.; Andrews, W.S. Emotional adaptation in children after liver transplantation. J. Pediatr. 1991, 119, 880–887. [Google Scholar] [CrossRef]
- Kirkley, A.; Griffin, S. Development of disease-specific quality of life measurement tools. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 1121–1128. [Google Scholar] [CrossRef]
- Ng, V.; Nicholas, D.B.; Dhawan, A.; Yazigi, N.A.; Ee, L.; Stormon, M.; Gilmour, S.; Schreiber, R.; Taylor, R.; Otley, A.R. Development and Validation of the Pediatric Liver Transplantation Quality of Life: A Disease-Specific Quality of Life Measure for Pediatric Liver Transplant Recipients. J. Pediatr. 2014, 165, 547–555.e7. [Google Scholar] [CrossRef]
- Konidis, S.V.; Hrycko, A.; Nightingale, S.; Renner, E.; Lilly, L.; Therapondos, G.; Fu, A.; Avitzur, Y.; Ng, V.L. Health-related quality of life in long-term survivors of paediatric liver transplantation. Paediatr. Child Health 2015, 20, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Miserachs, M.; Parmar, A.; Bakula, A.; Hierro, L.; D’Antiga, L.; Goldschmidt, I.; Debray, M.; McLin, V.A.; Casotti, V.; Pawłowska, J.; et al. Health-related quality of life in pre-adolescent liver transplant recipients with biliary atresia: A cross-sectional study. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Devine, K.A.; Reed, B.; Loiselle, K.A.; Simons, L.E.; Mee, L.L.; Blount, R.L. Predictors of Long-Term Health-Related Quality of Life in Adolescent Solid Organ Transplant Recipients. J. Pediatr. Psychol. 2011, 36, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Bucuvalas, J.; Hornung, R.W.; Krug, S.; Ryckman, F.C.; Atherton, H.; Alonso, M.P.; Balistreri, W.F.; Kotagal, U. Impact of liver transplantation on HRQOL in children less than 5 years old. Pediatr. Transplant. 2004, 8, 222–227. [Google Scholar] [CrossRef]
- Ohnemus, D.; Neighbors, K.; Rychlik, K.; Venick, R.S.; Bucuvalas, J.C.; Sundaram, S.S.; Ng, V.L.; Andrews, W.S.; Turmelle, Y.; Mazariegos, G.V.; et al. Health-Related Quality of Life and Cognitive Functioning in Pediatric Liver Transplant Recipients. Liver Transplant. 2019, 26, 45–56. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Manificat, S.; Dazord, A.; Cochat, P.; Morin, D.; Plainguet, F.; Debray, D. Quality of life of children and adolescents after kidney or liver transplantation: Child, parents and caregiver’s point of view. Pediatr. Transplant. 2003, 7, 228–235. [Google Scholar] [CrossRef]
- Nicholas, D.B.; Otley, A.R.; Taylor, R.; Dhawan, A.; Gilmour, S.; Ng, V.L. Experiences and barriers to Health-Related Quality of Life following liver transplantation: A qualitative analysis of the perspectives of pediatric patients and their parents. Health Qual. Life Outcomes 2010, 8, 150–158. [Google Scholar] [CrossRef]
- Alonso, E.M.; Martz, K.; Wang, D.; Yi, M.S.; Neighbors, K.; Varni, J.W.; Bucuvalas, J.C. The Studies of Pediatric Liver Transplantation (SPLIT) Functional Outcomes Group (FOG) Factors predicting health-related quality of life in pediatric liver transplant recipients in the functional outcomes group. Pediatr. Transplant. 2013, 17, 605–611. [Google Scholar] [CrossRef]
- Alonso, E.M.; Neighbors, K.; Barton, F.B.; McDiarmid, S.V.; Dunn, S.P.; Mazariegos, G.V.; Landgraf, J.M.; Bucuvalas, J. Studies of Pediatric Liver Transplant Research Group Health-related quality of life and family function following pediatric liver transplantation. Liver Transplant. 2008, 14, 460–468. [Google Scholar] [CrossRef]
- Parmar, A.; VanDriel, S.M.; Ng, V.L. Health-related quality of life after pediatric liver transplantation: A systematic review. Liver Transplant. 2017, 23, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W.; Seid, M.; Knight, T.S.; Uzark, K.; Szer, I.S. The PedsQLTM 4.0 Generic Core Scales: Sensitivity, Responsiveness, and Impact on Clinical Decision-Making. J. Behav. Med. 2002, 25, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.L.; Alonso, E.M.; Bucuvalas, J.C.; Cohen, G.; Limbers, C.A.; Varni, J.W.; Mazariegos, G.V.; Magee, J.; McDiarmid, S.V.; Anand, R.; et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: Report of the studies of pediatric liver transplantation experience. J. Pediatr. 2011, 160, 820–826.e3. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.; Uribe, M.; Hunter, B.; Monzon, P.; Ferrada, C.; Heine, C.; Auad, H. Health-related Quality of Life After Pediatric Liver Transplant: Single-Center Experience in Chile. Transplant. Proc. 2013, 45, 3728–3730. [Google Scholar] [CrossRef]
- Mager, D.R.; Marcon, M.; Brill, H.; Liu, A.; Radmanovich, K.; Mileski, H.; Nasser, R.; Alzaben, A.; Carroll, M.W.; Yap, J.; et al. Adherence to the Gluten-free Diet and Health-related Quality of Life in an Ethnically Diverse Pediatric Population with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 941–948. [Google Scholar] [CrossRef]
- Cholankeril, G.; Gonzalez, H.C.; Satapathy, S.; Gonzalez, S.A.; Hu, M.; Khan, M.A.; Yoo, E.R.; Li, A.A.; Kim, D.; Nair, S.; et al. Increased Waitlist Mortality and Lower Rate for Liver Transplantation in Hispanic Patients with Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2018, 16, 965–973.e2. [Google Scholar] [CrossRef]
- Kemmer, N.; Neff, G.W. Liver Transplantation in the Ethnic Minority Population: Challenges and Prospects. Dig. Dis. Sci. 2009, 55, 883–889. [Google Scholar] [CrossRef]
- Thammana, R.V.; Knechtle, S.J.; Romero, R.; Heffron, T.G.; Daniels, C.T.; Patzer, R.E. Racial and socioeconomic disparities in pediatric and young adult liver transplant outcomes. Liver Transplant. 2014, 20, 100–115. [Google Scholar] [CrossRef]
- Mager, D.R.; Al-Zaben, A.S.; Robert, C.; Gilmour, S.; Yap, J. Bone Mineral Density and Growth in Children Having Undergone Liver Transplantation with Corticosteroid-Free Immunosuppressive Protocol. J. Parenter. Enter. Nutr. 2015, 41, 632–640. [Google Scholar] [CrossRef]
- Scott, L.J.; McKeage, K.; Keam, S.J.; Plosker, G.L. Tacrolimus. Drugs 2003, 63, 1247–1297. [Google Scholar] [CrossRef]


| n | % | Median (IQR) | |
|---|---|---|---|
| Patient Characteristics at LTx | |||
| Sex | |||
| Male | 18 | 51.4 | |
| Female | 17 | 48.6 | |
| Age at LTx, yrs | 35 | 1.4 (0.8–3.3) | |
| Liver Disease Score | |||
| PELD | 34 | 97.1 | 15.4 (7.0–22.8) |
| MELD | 1 | 2.9 | 17.0 |
| Anthropometrics | |||
| Weight-z at LTx | 29 | −0.4 (−0.8–0.6) | |
| Height -z at LTx | 21 | −0.9 (−1.4–0.6) | |
| Graft Type | |||
| Living | 16 | 45.7 | |
| Cadaveric | 19 | 54.3 | |
| Indication for Transplant | |||
| Biliary Atresia | 19 | 54.3 | |
| Fulminant Liver Failure | 2 | 5.7 | |
| Metabolic Liver Diseases | 3 | 8.6 | |
| PSC | 3 | 8.6 | |
| Other | 8 | 22.9 | |
| Race | |||
| Caucasian | 12 | 34.3 | |
| Aboriginal | 3 | 8.6 | |
| Asian | 1 | 2.9 | |
| Black | 1 | 2.9 | |
| Unknown | 18 | 51.4 | |
| Patient Characteristics at PeLTQL | |||
| Age at initial PeLTQL, yrs | 35 | 11.6 (10.0–13.0) | |
| Age across all PeLTQL, yrs | 79 | 12.3 (10.8–13.8) | |
| < 12 | 36 | 45.6 | 10.5 (9.6–11.4) |
| ≥ 12 | 48 | 54.4 | 13.9 (13.0–14.8) |
| Anthropometrics at Initial PeLTQL | |||
| Weight-z | 29 | −0.2 (−0.4–0.6) | |
| Height-z | 29 | 0.5 (−0.50–0.8) | |
| Interval from LTx to all PeLTQL | 79 | 10.0 (7.6–12.3) | |
| Interval from LTx to initial PeLTQL | 35 | 8.8 (7.0–10.9) | |
| Medications | |||
| Number of medications | 49 | ||
| Tacrolimus Monotherapy † | 45 | 91.8 | 1.0 (1.0–2.0) |
| IS Polytherapy †,* | 4 | 8.2 | |
| No medication data available | 30 | ||
| Past Rejection (> 6mo) | 12 | 34.3 | |
| Acute | 8 | 22.9 | |
| Chronic | 4 | 11.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hager, A.; Mager, D.; Robert, C.; Nicholas, D.; Gilmour, S. Health-Related Quality of Life 10 Years after Liver Transplantation: A Longitudinal Retrospective Review. Diagnostics 2021, 11, 111. https://doi.org/10.3390/diagnostics11010111
Hager A, Mager D, Robert C, Nicholas D, Gilmour S. Health-Related Quality of Life 10 Years after Liver Transplantation: A Longitudinal Retrospective Review. Diagnostics. 2021; 11(1):111. https://doi.org/10.3390/diagnostics11010111
Chicago/Turabian StyleHager, Amber, Diana Mager, Cheri Robert, David Nicholas, and Susan Gilmour. 2021. "Health-Related Quality of Life 10 Years after Liver Transplantation: A Longitudinal Retrospective Review" Diagnostics 11, no. 1: 111. https://doi.org/10.3390/diagnostics11010111
APA StyleHager, A., Mager, D., Robert, C., Nicholas, D., & Gilmour, S. (2021). Health-Related Quality of Life 10 Years after Liver Transplantation: A Longitudinal Retrospective Review. Diagnostics, 11(1), 111. https://doi.org/10.3390/diagnostics11010111




