Biomarker Utility for Peripheral Artery Disease Diagnosis in Real Clinical Practice: A Prospective Study
Abstract
1. Introduction
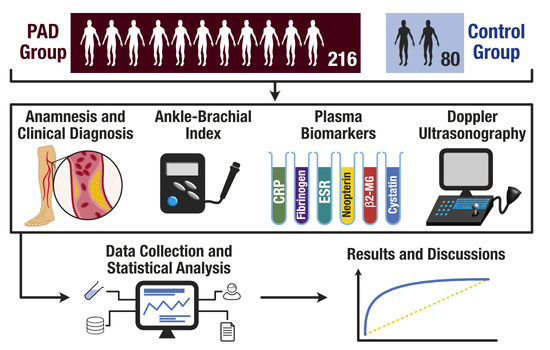
2. Materials and Methods
2.1. Statistical Analysis
2.2. Ethics Statement
3. Results
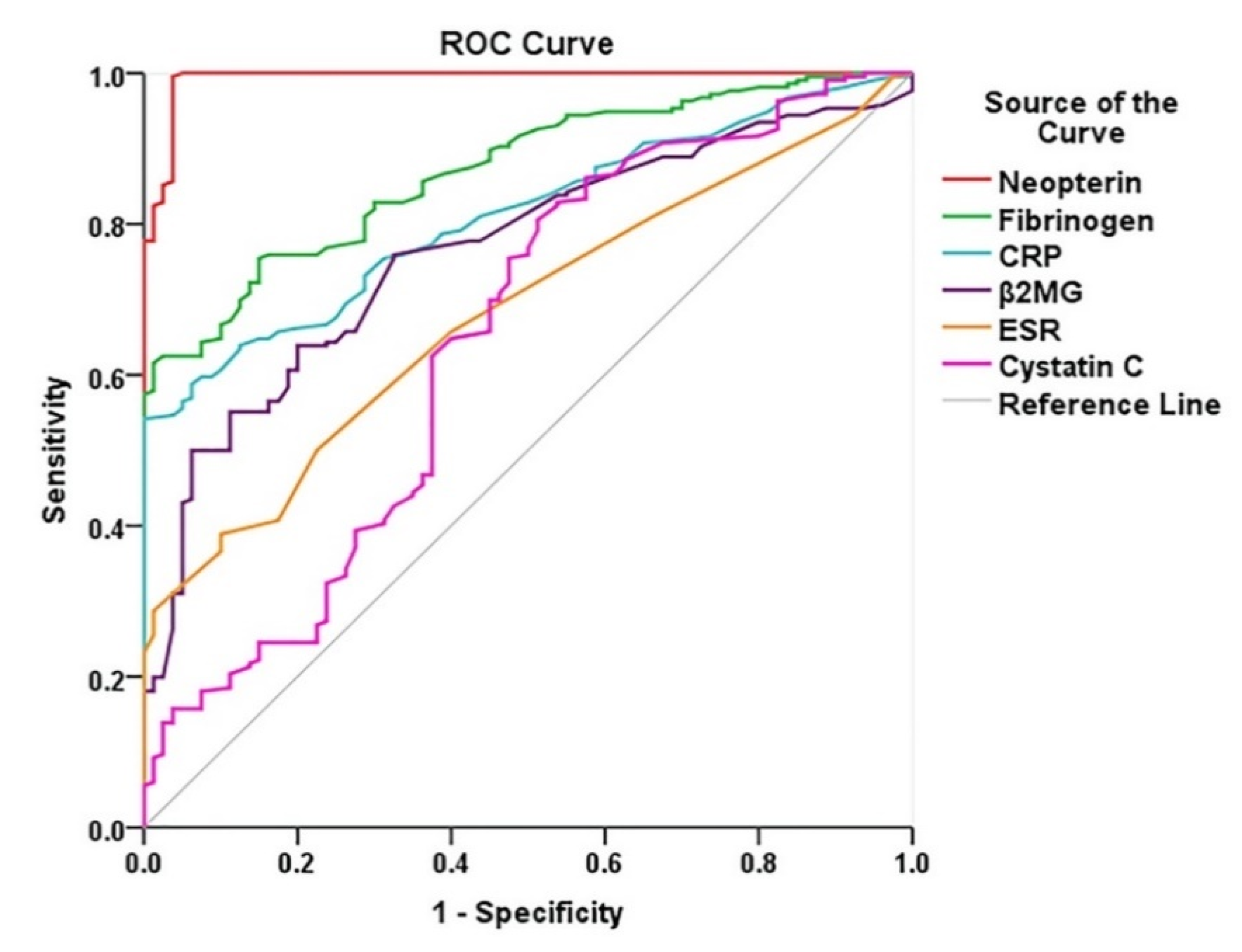
Diagnostic Performance of Biomarkers in PAD
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | ankle-brachial index |
| B2-MG | beta 2-microglobulin |
| CD | claudication distance |
| CRP | C-reactive protein |
| ESR | erythrocyte sedimentation rate |
| PAD | peripheral artery disease |
References
- Aboyans, V.; Ricco, J.; Bartelink, M.; Björck, M.; Brodmann, M.; Cohner, T.; Collet, J.; Czerny, M.; Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Ricco, J.; Aboyans, V.; Dick, F.; Kolh, P. Real Life Lessons in Peripheral Artery Disease—A Priority for Public Healthcare. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 5–6. [Google Scholar] [CrossRef]
- Lamberti, N.; López-Soto, P.; Guerzoni, F.; Napoli, N.; Gasbarro, V.; Zamboni, P.; Tsolaki, E.; Taddia, M.; Rodríguez-Borrego, M.; Manfredini, R.; et al. Changes in exercise capacity and risk of all-cause mortality in patients with peripheral artery disease: A 10-year retrospective cohort study. Intern. Emerg. Med. 2019, 15, 289–298. [Google Scholar] [CrossRef]
- Manfredini, F.; Lamberti, N.; Guerzoni, F.; Napoli, N.; Gasbarro, V.; Zamboni, P.; Mascoli, F.; Manfredini, R.; Basaglia, N.; Rodríguez-Borrego, M.; et al. Rehabilitative Exercise Reduced the Impact of Peripheral Artery Disease on Vascular Outcomes in Elderly Patients with Claudication: A Three-Year Single Center Retrospective Study. J. Clin. Med. 2019, 8, 210. [Google Scholar] [CrossRef]
- Harwood, A.; Totty, J.; Broadbent, E.; Smith, G.; Chetter, I. Quality of life in patients with intermittent claudication. Gefässchirurgie 2017, 22, 159–164. [Google Scholar] [CrossRef]
- Alabi, O.; Roos, M.; Landry, G.; Moneta, G. Quality-of-life assessment as an outcomes measure in critical limb ischemia. J. Vasc. Surg. 2017, 65, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Aber, A.; Lumley, E.; Phillips, P.; Woods, H.; Jones, G.; Michaels, J. Themes that Determine Quality of Life in Patients with Peripheral Arterial Disease: A Systematic Review. Patient Patient Cent. Outcomes Res. 2018, 11, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Pitcher, A.; Belch, J.; De Carlo, M.; Reinecke, H.; Baumgartner, I.; Mazzolai, L.; Aboyans, V. The year in cardiology: Aorta and peripheral circulation. Eur. Heart J. 2020, 41, 501–508b. [Google Scholar] [CrossRef]
- Conte, S.; Vale, P. Peripheral Arterial Disease. Heart Lung Circ. 2018, 27, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Moţa, E.; Popa, S.; Moţa, M.; Mitrea, A.; Penescu, M.; Tuţă, L.; Serafinceanu, C.; Hâncu, N.; Gârneaţă, L.; Verzan, C.; et al. Prevalence of chronic kidney disease and its association with cardio-metabolic risk factors in the adult Romanian population: The PREDATORR study. Int. Urol. Nephrol. 2015, 47, 1831–1838. [Google Scholar] [CrossRef]
- Mihai, B.; Petriș, A.; Ungureanu, D.; Lăcătușu, C. Insulin resistance and adipokine levels correlate with early atherosclerosis—A study in prediabetic patients. Open Med. 2014, 10, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.; Dormandy, J.; Nehler, M.; Harris, K.; Fowkes, F. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 2007, 33, S1–S75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shi, Y.; Wang, Y.; Li, X. Patterns of Disease Distribution of Lower Extremity Peripheral Arterial Disease. Angiology 2015, 66, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.; Aldasoro, M.; Victor, V.; Mauricio, M. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxidative Med. A Cell. Longev. 2019, 2019, 1–32. [Google Scholar] [CrossRef]
- Ruparelia, N.; Choudhury, R. Inflammation and atherosclerosis: What is on the horizon? Heart 2020, 106, 80–85. [Google Scholar] [CrossRef]
- Signorelli, S.; Anzaldi, M.; Libra, M.; Navolanic, P.; Malaponte, G.; Mangano, K.; Quattrocchi, C.; Di Marco, R.; Fiore, V.; Neri, S. Plasma Levels of Inflammatory Biomarkers in Peripheral Arterial Disease. Angiology 2016, 67, 870–874. [Google Scholar] [CrossRef]
- Raggi, P.; Genest, J.; Giles, J.; Rayner, K.; Dwivedi, G.; Beanlands, R.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Erren, M.; Reinecke, H.; Junker, R.; Fobker, M.; Schulte, H.; Schurek, J.; Kropf, J.; Kerber, S.; Breithardt, G.; Assmann, G.; et al. Systemic Inflammatory Parameters in Patients With Atherosclerosis of the Coronary and Peripheral Arteries. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2355–2363. [Google Scholar] [CrossRef]
- Ibaram, D. Role of C-Reactive Protein in Peripheral Arterial Disease. Int. J. Innov. Res. Med. Sci. 2018, 3, 2428–2432. [Google Scholar] [CrossRef]
- Paraskevas, K.; Baker, D.; Vrentzos, G.; Mikhailidis, D. The role of fibrinogen and fibrinolysis in peripheral arterial disease. Thromb. Res. 2008, 122, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.; Stampfer, M.; Rifai, N. Novel risk factors for systemic atherosclerosis. A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein (a), and standard cholesterol screening as predictors of peripheral arterial disease. J. Am. Med. Assoc. 2001, 285, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F. Fibrinogen and Peripheral Arterial Disease. Eur. Heart J. 1995, 16, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kiyani, M.; Jahan, S.; Kiyani, S.; Rehman, H.; Khan, L.; Iqbal, U. Erythrocyte sedimentation rate in diabetic and non-diabetic patients of cardiovascular disease. J. Teknol. Lab. 2019, 8, 18–22. [Google Scholar] [CrossRef]
- Erikssen, G.; Liestol, K.; Bjornholt, J.; Stormorken, H.; Thaulow, E.; Erikssen, J. Erythrocyte sedimentation rate: A possible marker of atherosclerosis and a strong predictor of coronary heart disease mortality. Eur. Heart J. 2000, 21, 1614–1620. [Google Scholar] [CrossRef]
- Pacileo, M.; Cirillo, P.; De Rosa, S.; Ucci, G.; Petrillo, G.; Musto D’Amore, S.; Sasso, L.; Maietta, P.; Spagnuolo, R.; Chiariello, M. The role of Neopterin in cardiovascular disease. Monaldi Arch. Chest Dis. 2016, 68, 68–73. [Google Scholar] [CrossRef][Green Version]
- De Rosa, S.; Cirillo, P.; Pacileo, M.; Petrillo, G.; D’Ascoli, G.; Maresca, F.; Ziviello, F.; Chiariello, M. Neopterin: From Forgotten Biomarker to Leading Actor in Cardiovascular Pathophysiology. Curr. Vasc. Pharmacol. 2011, 9, 188–199. [Google Scholar] [CrossRef]
- Signorelli, S.; Anzaldi, M.; Fiore, V.; Candido, S.; Di Marco, R.; Mangano, K.; Quattrocchi, C.; Neri, S. Neopterin: A potential marker in chronic peripheral arterial disease. Mol. Med. Rep. 2013, 7, 1855–1858. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Dai, W. Serum Cystatin C and the Risk of Coronary Heart Disease in Ethnic Chinese Patients with Normal Renal Function. Lab. Med. 2016, 47, 13–19. [Google Scholar] [CrossRef]
- Jin, Q.; Chen, Y.; Qian, G.; Tian, F.; Guo, J.; Jing, J. Serum cystatin C level and coronary artery plaque characteristics: An optical coherence tomography study. Eur. Heart J. Suppl. 2016, 18, A21–A26. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, T.; Yokokawa, H.; Fujibayashi, K.; Haniu, T.; Hisaoka, T.; Fukuda, H.; Naito, T. Association between high cystatin C levels and carotid atherosclerosis. World J. Cardiol. 2017, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.; Acosta, S.; Gottsäter, A.; Melander, O.; Engström, G.; Dakhel, A.; Zarrouk, M. Copeptin, B-type natriuretic peptide and cystatin C are associated with incident symptomatic PAD. Biomarkers 2019, 24, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Lamberti, N.; Ficarra, V.; Tsolaki, E.; Straudi, S.; Zamboni, P.; Basaglia, N.; Gasbarro, V. Biomarkers of Muscle Metabolism in Peripheral Artery Disease: A Dynamic NIRS-Assisted Study to Detect Adaptations Following Revascularization and Exercise Training. Diagnostics 2020, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Shirai, R.; Sato, K.; Yamashita, T.; Yamaguchi, M.; Okano, T.; Watanabe-Kominato, K.; Watanabe, R.; Matsuyama, T.; Ishibashi-Ueda, H.; Koba, S. Neopterin Counters Vascular Inflammation and Atherosclerosis. J. Am. Heart Assoc. 2018, 7, e007359. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, F.; Kullo, I. Novel markers of peripheral arterial disease. Vasc. Med. 2009, 14, 381–392. [Google Scholar] [CrossRef]
- Patel, M.; Conte, M.; Cutlip, D.; Dib, N.; Geraghty, P.; Gray, W.; Hiatt, W.; Ho, M.; Ikeda, K.; Ikeno, F.; et al. Evaluation and Treatment of Patients with Lower Extremity Peripheral Artery Disease. Consensus Definitions from Peripheral Academic Research Consortium (PARC). J. Am. Coll. Cardiol. 2015, 65, 931–941. [Google Scholar] [CrossRef]
- DeLong, E.; DeLong, D.; Clarke-Pearson, D. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Jaba, E.; Grama, A. Chapter 9, Analiza de corelatie si regresie. In Analiza Statistica cu SPSS sub Windows; Polirom: Iasi, Romania, 2004; pp. 232–268. [Google Scholar]
- Tzoulaki, I.; Murray, G.; Price, J.; Smith, F.; Lee, A.; Rumley, A.; Lowe, G.; Fowkes, F. Hemostatic Factors, Inflammatory Markers, and Progressive Peripheral Atherosclerosis. Am. J. Epidemiol. 2005, 163, 334–341. [Google Scholar] [CrossRef]
- Smith, F.; Lee, A.; Hau, C.; Rumley, A.; Lowe, G.; Fowkes, F. Plasma fibrinogen, haemostatic factors and prediction of peripheral arterial disease in the Edinburgh Artery Study. Blood Coagul. Fibrinolysis 2000, 11, 43–50. [Google Scholar] [CrossRef]
- Ünlü, Y.; Karapolat, S.; Karaca, Y.; Kızıltunç, A. Comparison of levels of inflammatory markers and hemostatic factors in the patients with and without peripheral arterial disease. Thromb. Res. 2006, 117, 357–364. [Google Scholar] [CrossRef]
- Igari, K.; Kudo, T.; Toyofuku, T.; Inoue, Y. Relationship of Inflammatory Biomarkers with Severity of Peripheral Arterial Disease. Int. J. Vasc. Med. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.; Thompson, S.; Williams, D.; AbuRahma, Z.; Grome, L.; Schlarb, H.; AbuRahma, A. Biochemical markers in patients with open reconstructions with peripheral arterial disease. Vascular 2016, 24, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Erlinger, T. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination survey, 1999–2000. ACC Curr. J. Rev. 2004, 13, 37–38. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kushner, I. Regulation of the Acute Phase Response by Cytokines. Perspect. Biol. Med. 1993, 36, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M. Abnormal laboratory results: Erythrocyte sedimentation rate and C-reactive protein. Aust. Prescr. 2015, 38, 93–94. [Google Scholar] [CrossRef]
- McDermott, M.; Green, D.; Greenland, P.; Liu, K.; Criqui, M.; Chan, C.; Guralnik, J.; Pearce, W.; Ridker, P.; Taylor, L.; et al. Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am. J. Cardiol. 2003, 92, 194–199. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.; Denenberg, J.; Knoke, J.; Ridker, P.; Fronek, A. Risk Factors for Progression of Peripheral Arterial Disease in Large and Small Vessels. Circulation 2006, 113, 2623–2629. [Google Scholar] [CrossRef]
- McDermott, M.; Guralnik, J.; Corsi, A.; Albay, M.; Macchi, C.; Bandinelli, S.; Ferrucci, L. Patterns of inflammation associated with peripheral arterial disease: The InCHIANTI study. Am. Heart J. 2005, 150, 276–281. [Google Scholar] [CrossRef]
- Kals, J.; Zagura, M.; Serg, M.; Kampus, P.; Zilmer, K.; Unt, E.; Lieberg, J.; Eha, J.; Peetsalu, A.; Zilmer, M. β2-microglobulin, a novel biomarker of peripheral arterial disease, independently predicts aortic stiffness in these patients. Scand. J. Clin. Lab. Investig. 2011, 71, 257–263. [Google Scholar] [CrossRef]
- Wilson, A.; Kimura, E.; Harada, R.; Nair, N.; Narasimhan, B.; Meng, X.; Zhang, F.; Beck, K.; Olin, J.; Fung, E.; et al. β2-Microglobulin as a Biomarker in Peripheral Arterial Disease. Circulation 2007, 116, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Wilson, A.; Zhang, F.; Harris, N.; Edwards, K.; Olin, J.; Cooke, J. A biomarker panel for peripheral arterial disease. Vasc. Med. 2008, 13, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Busti, C.; Migliacci, R.; Falcinelli, E.; Gresele, P. Plasma levels of β2-microglobulin, a biomarker of peripheral arterial disease, are not affected by maximal leg exercise in patients with intermittent claudication. Atherosclerosis 2009, 203, 38–40. [Google Scholar] [CrossRef]
- Real de Asúa, D.; Puchades, R.; García-Polo, I.; Suárez, C. A Study on the Relationship between Serum Beta 2-Microglobulin Levels, Underlying Chronic Kidney Disease, and Peripheral Arterial Disease in High-Vascular-Risk Patients. Int. Cardiovasc. Res. J. 2012, 6, 107–112. [Google Scholar] [PubMed]
- Rheeder, P.; Nel, L.; Meeuwes, F.; Van Schendel, M.; Meyer, P. Beta-2 microglobulin as a predictor of peripheral arterial disease in diabetes: The effect of estimated glomerular filtration. J. Endocrinol. Metab. Diabetes S. Afr. 2012, 17, 141–144. [Google Scholar] [CrossRef][Green Version]
- Madero, M.; Wassel, C.; Peralta, C.; Najjar, S.; Sutton-Tyrrell, K.; Fried, L.; Canada, R.; Newman, A.; Shlipak, M.; Sarnak, M. Cystatin C Associates with Arterial Stiffness in Older Adults. J. Am. Soc. Nephrol. 2009, 20, 1086–1093. [Google Scholar] [CrossRef]
- Liu, F.; Shen, J.; Zhao, J.; Zeng, H.; Li, L.; Zhao, J.; Lu, F.; Bao, Y.; Jia, W. Cystatin C: A Strong Marker for Lower Limb Ischemia in Chinese Type 2 Diabetic Patients? PLoS ONE 2013, 8, e66907. [Google Scholar] [CrossRef]
- Arpegård, J.; Östergren, J.; de Faire, U.; Hansson, L.; Svensson, P. Cystatin C—A marker of peripheral atherosclerotic disease? Atherosclerosis 2008, 199, 397–401. [Google Scholar] [CrossRef]
- Salgado, J.; Souza, F.; Salgado, B. How to understand the association between cystatin C levels and cardiovascular disease: Imbalance, counterbalance, or consequence? J. Cardiol. 2013, 62, 331–335. [Google Scholar] [CrossRef]
- Benndorf, R. Renal Biomarker and Angiostatic Mediator? Cystatin C as a Negative Regulator of Vascular Endothelial Cell Homeostasis and Angiogenesis. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Joosten, M.; Pai, J.; Bertoia, M.; Gansevoort, R.; Bakker, S.; Cooke, J.; Rimm, E.; Mukamal, K. β2-Microglobulin, Cystatin C, and Creatinine and Risk of Symptomatic Peripheral Artery Disease. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Zonda, G.; Zonda, R.; Cernomaz, A.; Paduraru, L.; Grigoriu, B. Endocan serum concentration in uninfected newborn infants. J. Infect. Dev. Ctries. 2019, 13, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Barani, J.; Nilsson, J.; Mattiasson, I.; Lindblad, B.; Gottsäter, A. Inflammatory mediators are associated with 1-year mortality in critical limb ischemia. J. Vasc. Surg. 2005, 42, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Brevetti, G.; Schiano, V.; Chiariello, M. Endothelial dysfunction: A key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 2008, 197, 1–11. [Google Scholar] [CrossRef]
- Igari, K.; Kudo, T.; Toyofuku, T.; Inoue, Y. The Relationship between Endothelial Dysfunction and Endothelial Cell Markers in Peripheral Arterial Disease. PLoS ONE 2016, 11, e0166840. [Google Scholar] [CrossRef]
- Brevetti, G.; Silvestro, A.; Di Giacomo, S.; Bucur, R.; Di Donato, A.; Schiano, V.; Scopacasa, F. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J. Vasc. Surg. 2003, 38, 374–379. [Google Scholar] [CrossRef]
- Brevetti, G.; Silvestro, A.; Schiano, V.; Chiariello, M. Endothelial Dysfunction and Cardiovascular Risk Prediction in Peripheral Arterial Disease. Circulation 2003, 108, 2093–2098. [Google Scholar] [CrossRef]


| Characteristic | Total (n = 296) | PAD (n = 216) | No PAD (n = 80) | p-Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 69 (15) | 69 (15) | 68 (16) | 0.709 |
| Gender, N (%) | 0.773 | |||
| Women | 56 (18.9) | 40 (18.5) | 16 (20) | |
| Men | 240 (81.1) | 176 (81.5) | 64 (80) | |
| Residence, N (%) | 0.133 | |||
| Urban | 117 (39.5) | 91 (42.1) | 26 (32.5) | |
| Rural | 179 (60.5) | 125 (57.9) | 54 (67.5) | |
| Cigarette smoking, N (%) | 0.862 | |||
| Current smoker | 73 (24.7) | 53 (24.5) | 20 (25) | |
| Ex-smoker | 183 (61.8) | 135 (62.5) | 48 (60) | |
| Non-smoker | 40 (13.5) | 28 (13) | 12 (15) | |
| BMI, kg/m2, median (IQR) | 27.14 (6.46) | 27.07 (6.25) | 27.49 (7.04) | 0.628 |
| Hypertension, N (%) | 274 (92.6) | 198 (91.7) | 76 (95) | 0.332 |
| Diabetes mellitus, N (%) | 119 (40.2) | 89 (41.2) | 30 (37.5) | 0.564 |
| Dyslipidemia, N (%) | 278 (93.9) | 204 (94.4) | 74 (92.5) | 0.535 |
| Coronary artery disease, N (%) | 203 (68.6) | 145 (67.1) | 58 (72.5) | 0.378 |
| Heart failure, N (%) | 212 (71.6) | 152 (70.4) | 60 (75) | 0.433 |
| CD, median (IQR) | 200 (18.25–517) | 140 (10–240) | 800 (600–945) | <0.0001 |
| ABI, median (IQR) | 0.76 (0.49–0.99) | 0.67 (0.36–1.19) | 1.025(1.01–1.12) | <0.0001 |
| Variables | Total (n = 296) | PAD (n = 216) | No PAD (n = 80) | p-Value |
|---|---|---|---|---|
| CRP, mg/dL | 0.35 (0.15–0.9) | 0.52 (0.22–1.4) | 0.15 (0.08–0.27) | <0.0001 |
| Fibrinogen, mg/dL | 386.5 (344–454.75) | 417 (367–467) | 355.5 (302.25–362) | <0.0001 |
| ESR, mm/1 h | 6 (4–10) | 6.5 (4–13) | 5 (3–6) | <0.0001 |
| Neopterin, nmol/L | 11.75 (9.61–21.05) | 13.15 (11.17–28.42) | 5.58 (3.91–8.11) | <0.0001 |
| B2-MG, mg/L | 2.13 (1.6–3.63) | 2.62 (1.8–4) | 1.6 (1.3–1.94) | 0.046 |
| Cystatin C, mg/L | 1.16 (0.93–1.48) | 1.19 (1.01–1.49) | 0.99 (0.86–1.36) | 0.001 |
| Variables | CRP | Fibrinogen | ESR | Neopterin | B2-MG | Cystatin C |
|---|---|---|---|---|---|---|
| CRP | 1.00 | 0.557 * | 0.495 * | 0.759 * | 0.310 * | 0.233 * |
| Fibrinogen | 0.557 * | 1.00 | 0.501 * | 0.673 * | 0.303 * | 0.242 * |
| ESR | 0.495 * | 0.501 * | 1.00 | 0.496 * | 0.186 * | 0.234 * |
| Neopterin | 0.759 * | 0.673 * | 0.496 * | 1.00 | 0.350 * | 0.293 * |
| B2-MG | 0.310 * | 0.303 * | 0.186 * | 0.350 * | 1.00 | 0.192 * |
| Cystatin C | 0.233 * | 0.242 * | 0.234 * | 0.293 * | 0.192 * | 1.00 |
| CD | −0.765 * | −0.765 * | −0.503 * | −0.900 * | −0.353 * | −0.293 * |
| ABI | −0.761 * | −0.732 * | −0.505 * | −0.900 * | −0.377 * | −0.302 |
| No of arteries with >50% stenosis | 0.528 * | 0.508 * | 0.319 * | 0.524 * | 0.299 * | 0.354 * |
| No of arteries with >70% stenosis | 0.663 * | 0.458 * | 0.434 * | 0.693 * | 0.162 * | 0.269 * |
| Variables | AUC | p-Value | 95% CI for AUC |
|---|---|---|---|
| Neopterin, nmol/L | 0.993 | <0.0001 | 0.985–1.000 |
| Fibrinogen, mg/dL | 0.870 | <0.0001 | 0.831–0.910 |
| CRP, mg/dL | 0.811 | <0.0001 | 0.763–0.858 |
| B2-MG, mg/L | 0.765 | <0.0001 | 0.709–0.821 |
| ESR, mm/1 h | 0.682 | <0.0001 | 0.620–0.744 |
| Cystatin C, mg/L | 0.645 | <0.0002 | 0.570–0.721 |
| Variables | Coefficient (B) | SE | Exp(B)/Odds Ratio | 95% Cl for Exp(B) | p-Value |
|---|---|---|---|---|---|
| Neopterin, nmol/L | 3.771 | 0.964 | 43.441 | 6.563–287.518 | <0.0001 |
| Fibrinogen, mg/dL | 0.03 | 0.004 | 1.03 | 1.022–1.039 | <0.0001 |
| CRP, mg/dL | 4.891 | 0.898 | 133.135 | 22.904–773.89 | <0.0001 |
| B2-MG, mg/L | 0.874 | 0.166 | 2.396 | 1.73–3.317 | <0.0001 |
| ESR, mm/1 h | 0.186 | 0.041 | 1.205 | 1.112–1.306 | <0.0001 |
| Cystatin C, mg/L | 1.297 | 0.382 | 3.658 | 1.73–7.734 | 0.001 |
| Variables | Coefficient (B) | SE | Exp(B)/Odds Ratio | 95% Cl for Exp(B) | p-Value |
|---|---|---|---|---|---|
| Intercept (Constant) | −62.177 | 16.764 | - | - | <0.0001 |
| Neopterin, nmol/L | 4.728 | 1.331 | 113.113 | 8.329–1536.102 | <0.0001 |
| Fibrinogen, mg/dL | 0.033 | 0.014 | 1.034 | 1.005–1.063 | 0.019 |
| Cystatin C, mg/L | 4.409 | 2.098 | 82.155 | 1.345–5016.741 | 0.036 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceasovschih, A.; Sorodoc, V.; Onofrei, V.; Tesloianu, D.; Tuchilus, C.; Anisie, E.; Petris, A.; Statescu, C.; Jaba, E.; Stoica, A.; et al. Biomarker Utility for Peripheral Artery Disease Diagnosis in Real Clinical Practice: A Prospective Study. Diagnostics 2020, 10, 723. https://doi.org/10.3390/diagnostics10090723
Ceasovschih A, Sorodoc V, Onofrei V, Tesloianu D, Tuchilus C, Anisie E, Petris A, Statescu C, Jaba E, Stoica A, et al. Biomarker Utility for Peripheral Artery Disease Diagnosis in Real Clinical Practice: A Prospective Study. Diagnostics. 2020; 10(9):723. https://doi.org/10.3390/diagnostics10090723
Chicago/Turabian StyleCeasovschih, Alexandr, Victorita Sorodoc, Viviana Onofrei (Aursulesei), Dan Tesloianu, Cristina Tuchilus, Ecaterina Anisie, Antoniu Petris, Cristian Statescu, Elisabeta Jaba, Alexandra Stoica, and et al. 2020. "Biomarker Utility for Peripheral Artery Disease Diagnosis in Real Clinical Practice: A Prospective Study" Diagnostics 10, no. 9: 723. https://doi.org/10.3390/diagnostics10090723
APA StyleCeasovschih, A., Sorodoc, V., Onofrei, V., Tesloianu, D., Tuchilus, C., Anisie, E., Petris, A., Statescu, C., Jaba, E., Stoica, A., Grigorescu, E.-D., Jaba, I. M., & Sorodoc, L. (2020). Biomarker Utility for Peripheral Artery Disease Diagnosis in Real Clinical Practice: A Prospective Study. Diagnostics, 10(9), 723. https://doi.org/10.3390/diagnostics10090723