Acute Myocarditis-Like Episode in a Curly-Haired Young Boy—Red Flags for Familial Arrhythmogenic Cardiomyopathy
Abstract
1. Introduction
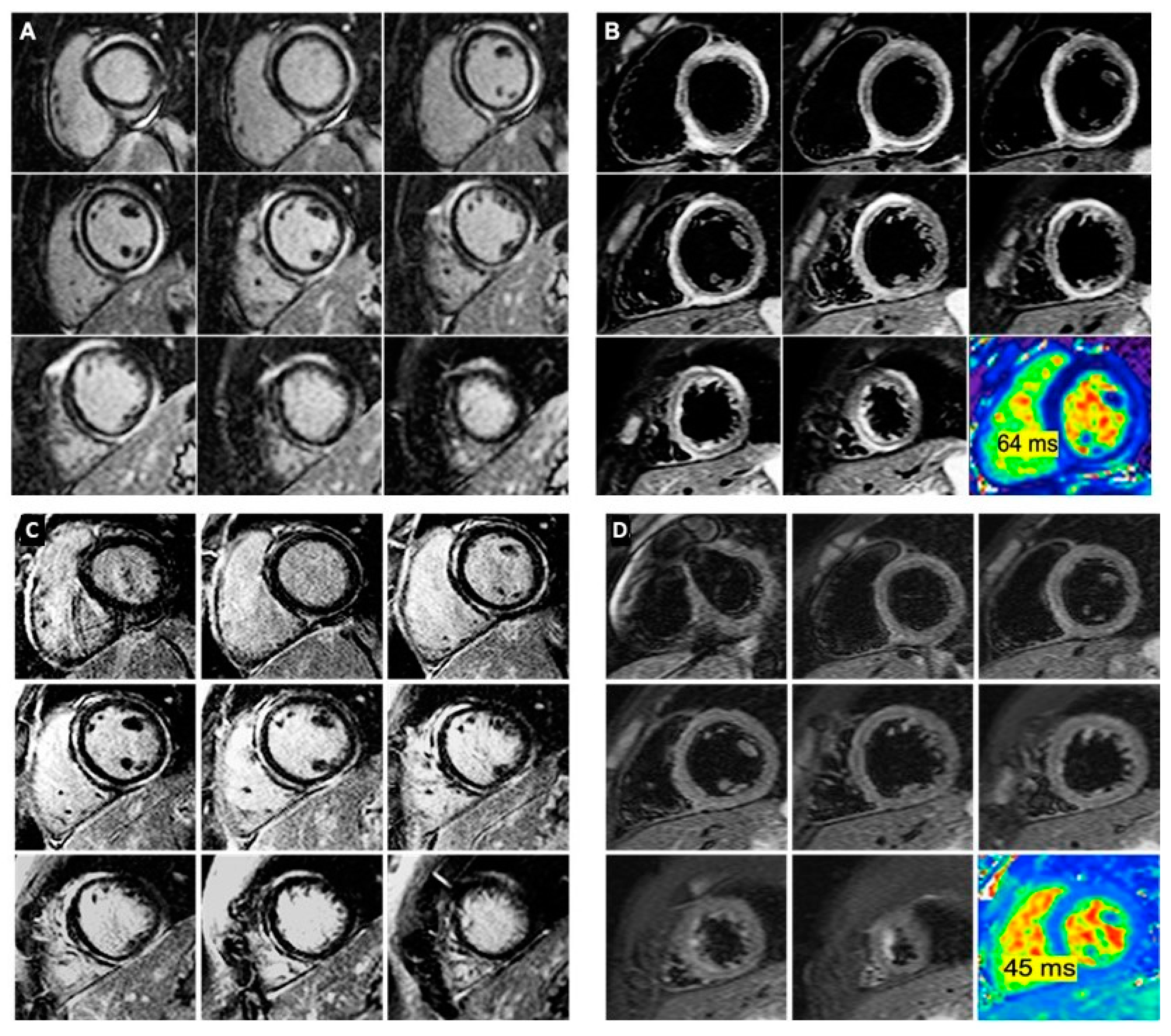
2. Case Reports
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.C.; Daubert, J.P.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019, 16, e301–e372. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.P.J.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation 2010, 121, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ven, J.P.G.; Sadighy, Z.; Buechel, E.R.V.; Sarikouch, S.; Robbers-Visser, D.; Kellenberger, C.J.; Kaiser, T.; Beerbaum, P.; Boersma, E.; Helbing, W.A. Multicentre reference values for cardiac magnetic resonance imaging derived ventricular size and function for children aged 0–18 years. Eur. Heart J. Cardiovasc. Imaging 2019, 21, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Maruthappu, T.; Posafalvi, A.; Castelletti, S.; Delaney, P.; Syrris, P.; O’Toole, E.; Green, K.; Elliott, P.; Lambiase, P.; Tinker, A.; et al. Loss-of-function desmoplakin I and II mutations underlie dominant arrhythmogenic cardiomyopathy with a hair and skin phenotype. Br. J. Dermatol. 2019, 180, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Ovaert, C.; Khraiche, D.; Boddaert, N.; Bonnet, D.; Raimondi, F. Myocardial inflammation detected by cardiac MRI in Arrhythmogenic right ventricular cardiomyopathy: A paediatric case series. Int. J. Cardiol. 2018, 271, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Protonotarios, N.; Tsatsopoulou, A.; Papachristou, P.; Sfendouraki, E.; Papadopoulos, G. Naxos disease evolution mimicking acute myocarditis: The role of cardiovascular magnetic resonance imaging. Int. J. Cardiol. 2013, 166, e14–e15. [Google Scholar] [CrossRef] [PubMed]
- Protonotarios, A.; Elliott, P.M. Arrhythmogenic right ventricular cardiomyopathy as a hidden cause of paediatric myocarditis presentation. Int. J. Cardiol. 2018, 271, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Piriou, N.; Marteau, L.; Kyndt, F.; Serfaty, J.M.; Toquet, C.; Le Gloan, L.; Warin-Fresse, K.; Guijarro, D.; Le Tourneau, T.; Conan, E.; et al. Familial screening in case of acute myocarditis reveals inherited arrhythmogenic left ventricular cardiomyopathies. ESC Heart Fail. 2020, 7, 1520–1533. [Google Scholar] [CrossRef] [PubMed]
- López-Ayala, J.M.; Pastor-Quirante, F.; González-Carrillo, J.; López-Cuenca, D.; Sánchez-Muñoz, J.J.; Oliva-Sandoval, M.J.; Gimeno, J.R. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm 2015, 12, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Corrado, D.; Valente, M.; Angelini, A.; Nava, A.; Thiene, G. Arrhythmogenic right ventricular cardiomyopathy: Dysplasia, dystrophy or myocarditis? J. Am. Coll. Cardiol. 1996, 27, 394. [Google Scholar] [CrossRef][Green Version]
- Smith, E.D.; Lakdawala, N.K.; Papoutsidakis, N.; Aubert, G.; Mazzanti, A.; McCanta, A.C.; Agarwal, P.P.; Arscott, P.; Dellefave-Castillo, L.M.; Vorovich, E.E.; et al. Desmoplakin Cardiomyopathy, a Fibrotic and Inflammatory Form of Cardiomyopathy Distinct From Typical Dilated or Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation 2020, 141, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.; Eiros, R.; Nakou, E.; Moura-Ferreira, S.; Treibel, T.; Captur, G.; Akhtar, M.M.; Protonotarios, A.; Gossios, T.D.; Savvatis, K.; et al. Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: A comprehensive genotype-imaging phenotype study. Eur. Heart J. Cardiovasc. Imaging 2019, 21, 326–336. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pătru, A.E.; Onciul, S.; Sturzu, A.; Cinteză, E.; Gima, E.; Popescu, B.A.; Chevalier, P.; Jurcuț, R. Acute Myocarditis-Like Episode in a Curly-Haired Young Boy—Red Flags for Familial Arrhythmogenic Cardiomyopathy. Diagnostics 2020, 10, 651. https://doi.org/10.3390/diagnostics10090651
Pătru AE, Onciul S, Sturzu A, Cinteză E, Gima E, Popescu BA, Chevalier P, Jurcuț R. Acute Myocarditis-Like Episode in a Curly-Haired Young Boy—Red Flags for Familial Arrhythmogenic Cardiomyopathy. Diagnostics. 2020; 10(9):651. https://doi.org/10.3390/diagnostics10090651
Chicago/Turabian StylePătru, Alina Elena, Sebastian Onciul, Adrian Sturzu, Eliza Cinteză, Eleonora Gima, Bogdan A. Popescu, Philippe Chevalier, and Ruxandra Jurcuț. 2020. "Acute Myocarditis-Like Episode in a Curly-Haired Young Boy—Red Flags for Familial Arrhythmogenic Cardiomyopathy" Diagnostics 10, no. 9: 651. https://doi.org/10.3390/diagnostics10090651
APA StylePătru, A. E., Onciul, S., Sturzu, A., Cinteză, E., Gima, E., Popescu, B. A., Chevalier, P., & Jurcuț, R. (2020). Acute Myocarditis-Like Episode in a Curly-Haired Young Boy—Red Flags for Familial Arrhythmogenic Cardiomyopathy. Diagnostics, 10(9), 651. https://doi.org/10.3390/diagnostics10090651






