Simulation-Based Virtual-Reality Patient-Specific Rehearsal Prior to Endovascular Procedures: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Process
2.3. Data Collection
2.4. Kirkpatrick’s Model Designed to Objectively Measure the Effectiveness of Training
2.5. Bias Assessment
3. Results
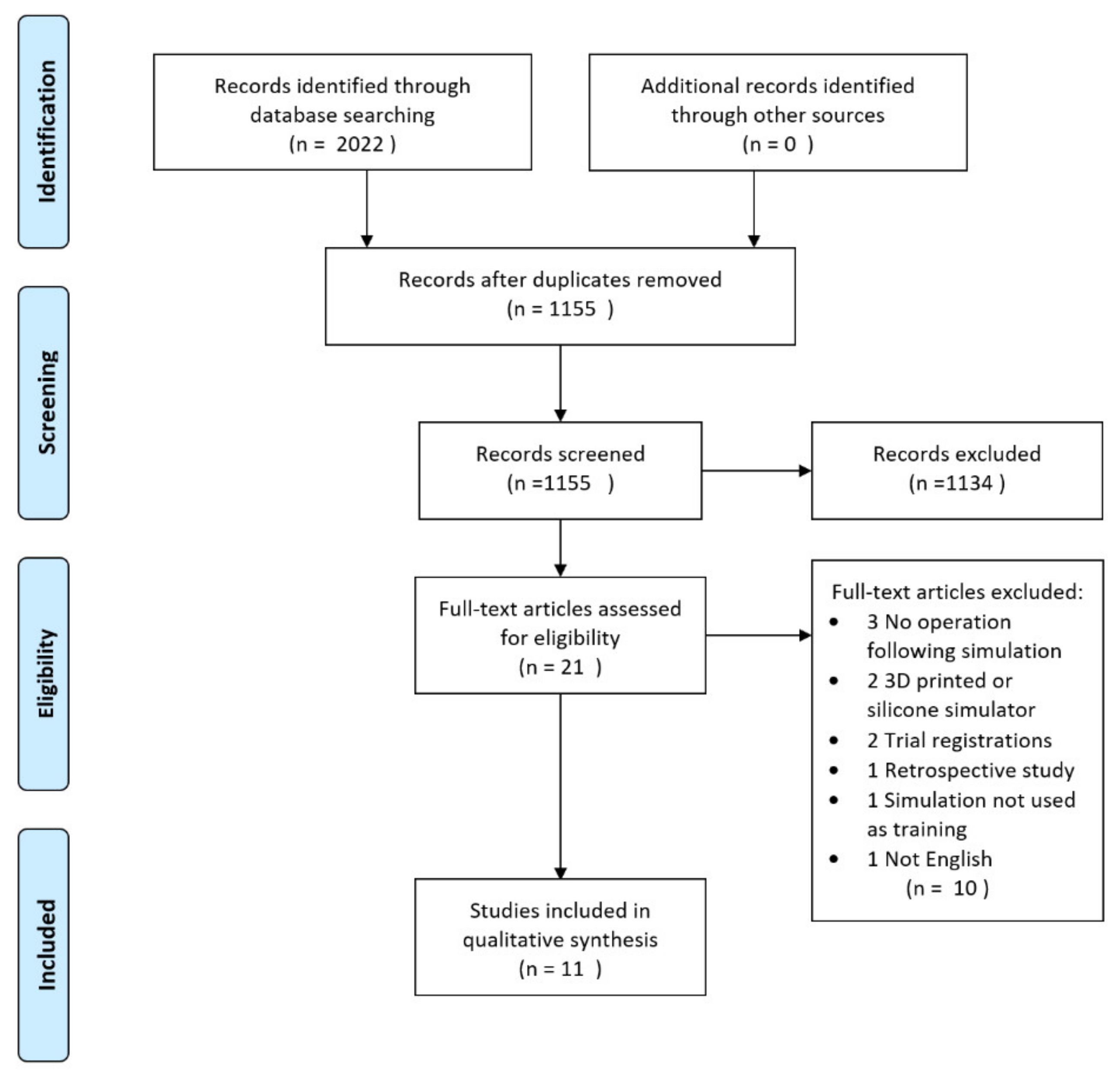
3.1. Study Selection
3.2. Study Characteristics
3.3. Study Findings
3.4. Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allerton, D. Principles of Flight Simulation; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0-470-68219-7. [Google Scholar]
- Landman, A.; van Oorschot, P.; van Paassen, M.M.R.; Groen, E.L.; Bronkhorst, A.W.; Mulder, M. Training Pilots for Unexpected Events: A Simulator Study on the Advantage of Unpredictable and Variable Scenarios. Hum. Factors 2018, 60, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Goolsarran, N.; Hamo, C.E.; Lane, S.; Frawley, S.; Lu, W.-H. Effectiveness of an interprofessional patient safety team-based learning simulation experience on healthcare professional trainees. BMC Med. Educ. 2018, 18, 192. [Google Scholar] [CrossRef]
- Ruikar, D.D.; Hegadi, R.S.; Santosh, K.C. A Systematic Review on Orthopedic Simulators for Psycho-Motor Skill and Surgical Procedure Training. J. Med. Syst. 2018, 42, 168. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A. Virtual Reality and Simulation in Neurosurgical Training. World Neurosurg. 2017, 106, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Van Cleynenbreugel, B.S.E.P.; Gözen, A.S.; Tokas, T. The value of simulation-based training in the path to laparoscopic urological proficiency. Curr. Opin. Urol. 2017, 27, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, F.; Thomsen, A.S.S.; Nayahangan, L.J.; Konge, L. Surgical simulation: Current practices and future perspectives for technical skills training. Med. Teach. 2018, 40, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Konge, L.; Lonn, L. Simulation-based training of surgical skills. Perspect. Med. Educ. 2016, 5, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Konge, L.; Clementsen, P.F.; Ringsted, C.; Minddal, V.; Larsen, K.R.; Annema, J.T. Simulator training for endobronchial ultrasound: A randomised controlled trial. Eur. Respir. J. 2015, 46, 1140–1149. [Google Scholar] [CrossRef]
- Willaert, W.I.M.; Aggarwal, R.; Van Herzeele, I.; Cheshire, N.J.; Vermassen, F.E. Recent advancements in medical simulation: Patient-specific virtual reality simulation. World J. Surg. 2012, 36, 1703–1712. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Covidence-Better Systematic Review Management. Available online: http://www.covidence.org/home (accessed on 18 March 2020).
- Desender, L.; Van Herzeele, I.; Lachat, M.; Duchateau, J.; Bicknell, C.; Teijink, J.; Heyligers, J.; Vermassen, F.; PAVLOV Study Group. A Multicentre Trial of Patient specific Rehearsal Prior to EVAR: Impact on Procedural Planning and Team Performance. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kurt, S. "Kirkpatrick Model: Four Levels of Learning Evaluation," in Educational Technology, October 24, 2016. Available online: https://educationaltechnology.net/kirkpatrick-model-four-levels-learning-evaluation/ (accessed on 13 July 2020).
- Reed, D.A.; Cook, D.A.; Beckman, T.J.; Levine, R.B.; Kern, D.E.; Wright, S.M. Association between funding and quality of published medical education research. JAMA 2007, 298, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Desender, L.M.; Van Herzeele, I.; Lachat, M.L.; Rancic, Z.; Duchateau, J.; Rudarakanchana, N.; Bicknell, C.D.; Heyligers, J.M.M.; Teijink, J.A.W.; Vermassen, F.E.; et al. Patient-specific Rehearsal Before EVAR: Influence on Technical and Nontechnical Operative Performance. A Randomized Controlled Trial. Ann. Surg. 2016, 264, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Wooster, M.; Doyle, A.; Hislop, S.; Glocker, R.; Armstrong, P.; Singh, M.; Illig, K.A. REHEARSAL Using Patient-Specific Simulation to Improve Endovascular Efficiency. Vasc. Endovascular Surg. 2018, 52, 169–172. [Google Scholar] [CrossRef]
- Våpenstad, C.; Lamøy, S.M.; Aasgaard, F.; Manstad-Hulaas, F.; Aadahl, P.; Søvik, E.; Stensæth, K.H. Influence of patient-specific rehearsal on operative metrics and technical success for endovascular aneurysm repair. Minim. Invasive Ther. Allied Technol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hislop, S.J.; Hedrick, J.H.; Singh, M.J.; Rhodes, J.M.; Gillespie, D.L.; Johansson, M.; Illig, K.A. Simulation case rehearsals for carotid artery stenting. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 750–754. [Google Scholar] [CrossRef]
- Roguin, A.; Beyar, R. Real case virtual reality training prior to carotid artery stenting. Catheter. Cardiovasc. Interv. 2010, 75, 279–282. [Google Scholar] [CrossRef]
- Willaert, W.; Aggarwal, R.; Bicknell, C.; Hamady, M.; Darzi, A.; Vermassen, F.; Cheshire, N.; European Virtual Reality Endovascular Research Team (EVEResT). Patient-specific simulation in carotid artery stenting. J. Vasc. Surg. 2010, 52, 1700–1705. [Google Scholar] [CrossRef]
- Willaert, W.I.M.; Aggarwal, R.; Van Herzeele, I.; Plessers, M.; Stroobant, N.; Nestel, D.; Cheshire, N.; Vermassen, F. Role of patient-specific virtual reality rehearsal in carotid artery stenting. Br. J. Surg. 2012, 99, 1304–1313. [Google Scholar] [CrossRef]
- Desender, L.; Rancic, Z.; Aggarwal, R.; Duchateau, J.; Glenck, M.; Lachat, M.; Vermassen, F.; Van Herzeele, I.; EVEREST (European Virtual Reality Endovascular RESearch Team). Patient-specific rehearsal prior to EVAR: A pilot study. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 639–647. [Google Scholar] [CrossRef]
- Pakeliani, D.; Bleuler, A.; Chaykovska, L.; Veith, F.J.; Criado, F.J.; Lachat, M.; Pfammatter, T.; Pecoraro, F. Patient-Specific Rehearsal Feasibility Before Endovascular Repair of Ruptured Abdominal Aortic Aneurysm. J. Endovasc. Ther. 2019, 26, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Desender, L.M.; Van Herzeele, I.; Rancic, Z.; Bicknell, C.; Zairis, I.; Vermassen, F.E.; Rundback, J.H. Patient-Specific Simulation of Endovascular Thoracic Aortic Repair: Initial Experience. Ann. Thorac. Surg. 2017, 104, 336–341. [Google Scholar] [CrossRef][Green Version]
- Sørensen, J.L.; Østergaard, D.; LeBlanc, V.; Ottesen, B.; Konge, L.; Dieckmann, P.; Van der Vleuten, C. Design of simulation-based medical education and advantages and disadvantages of in situ simulation versus off-site simulation. BMC Med. Educ. 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.A.; Hatala, R.; Brydges, R.; Zendejas, B.; Szostek, J.H.; Wang, A.T.; Erwin, P.J.; Hamstra, S.J. Technology-enhanced simulation for health professions education: A systematic review and meta-analysis. JAMA 2011, 306, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Yiasemidou, M.; Glassman, D.; Jayne, D.; Miskovic, D. Is patient-specific pre-operative preparation feasible in a clinical environment? A systematic review and meta-analysis. Comput. Assist. Surg. 2018, 23, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Berger, V.W.; Bears, J.D. When can a clinical trial be called “randomized”? Vaccine 2003, 21, 468–472. [Google Scholar] [CrossRef]
- Rosenberger, W.F.; Uschner, D.; Wang, Y. Randomization: The forgotten component of the randomized clinical trial. Stat. Med. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Parab, S.; Bhalerao, S. Study designs. Int. J. Ayurveda Res. 2010, 1, 128–131. [Google Scholar] [CrossRef]
- Lim, C.-Y.; In, J. Randomization in clinical studies. Korean J. Anesthesiol. 2019, 72, 221–232. [Google Scholar] [CrossRef]
- Case, L.D.; Ambrosius, W.T. Power and sample size. Methods Mol. Biol. 2007, 404, 377–408. [Google Scholar] [CrossRef]
- Harden, M.; Friede, T. Sample size calculation in multi-centre clinical trials. BMC Med. Res. Methodol. 2018, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.; Østergaard, M.L.; Nielsen, M.B.; Konge, L.; Nielsen, K.R. Standardised assessment of competence in Focused Assessment with Sonography for Trauma. Acta Anaesthesiol. Scand. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kahr Rasmussen, N.; Andersen, T.T.; Carlsen, J.; Østergaard, M.L.; Konge, L.; Albrecht-Beste, E.; Nielsen, M.B. Simulation-Based Training of Ultrasound-Guided Procedures in Radiology-A Systematic Review. Ultraschall Med. 2019, 40, 584–602. [Google Scholar] [CrossRef]
- Østergaard, M.L.; Nielsen, K.R.; Albrecht-Beste, E.; Konge, L.; Nielsen, M.B. Development of a reliable simulation-based test for diagnostic abdominal ultrasound with a pass/fail standard usable for mastery learning. Eur. Radiol. 2018, 28, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Borgersen, N.J.; Naur, T.M.H.; Sørensen, S.M.D.; Bjerrum, F.; Konge, L.; Subhi, Y.; Thomsen, A.S.S. Gathering Validity Evidence for Surgical Simulation: A Systematic Review. Ann. Surg. 2018, 267, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Coyer, F.M.; Nash, R. Kirkpatrick’s Evaluation of Simulation and Debriefing in Health Care Education: A Systematic Review. J. Nurs. Educ. 2018, 57, 393–398. [Google Scholar] [CrossRef] [PubMed]

| First author (No. of Perform. MDs) | No. of Patients | Procedure Time (min) | Fluoroscopy Time (min) | Contrast Volume (mL) | No. of Angiograms | Other End Points | Kirkpatrick’s Level | |
|---|---|---|---|---|---|---|---|---|
| CAS | ||||||||
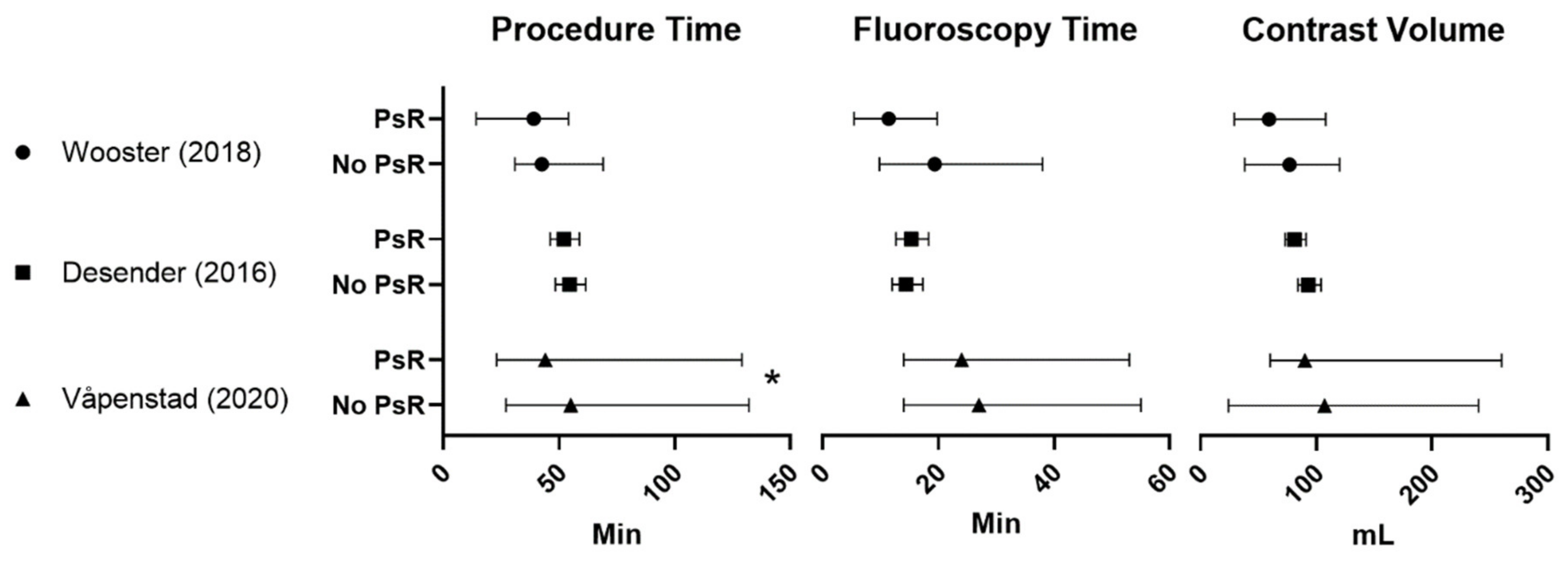
| Wooster (2018) [17] (3 MDs) | 6 PsR | 31.9 (14.2–54) + | 11.4 (5.4–19.8) | 59.2 (29–108) + | N/A | 3 | ||
| 9 controls | 42.5 (30.9–69) + | 19.4 (9.8–38) + | 76.9 (38–120) + | |||||
| EVAR | ||||||||
| Desender (2016) [16] (99 MDs) a and Desender (2017) [13] b | 50 PsR | 52.1 (46.2–58.8) + | 15.3 (12.7–18.3) + | 81 (73–91) + | 6.5 (5.9–7.2) + | Proximal landing zone: −23% *,+ Distal landing zone: −21% *,+ | Reduction in minor errors: −26% * Reduction in major errors: −76% * Reduction in errors causing delay: −27% * | 4 |
| 50 controls | 54.6 (48.4–61.6) + | 14.4 (12–17.3) + | 93 (84–104) + | 7.5 (6.7–8.2) + | Technical success rates: Primary: PsR 82% vs. control 78% Ass. Primary PsR 94% vs. control 86% | |||
| Våpenstad (2020) [18] (16 MDs) c | 30 PsR | 44 (23–129) * | 24 (14–53) | 90 (60–260) | 7 (4–18) | Technical success rates: Primary: PsR 93% vs. control 77% Ass. Primary: PsR 3,3% vs. control 13% | 3 | |
| 30 controls | 55 (27–132) | 27 (14–55) | 107 (24–240) | 7 (4–20) | ||||
| First Author (No. of Perform. MDs) | No. of Patients | Procedure Time (min) | Fluoroscopy Time (min) | Contrast Volume (mL) | No. of Angiograms | Realism | Kirkpatrick’s Level |
|---|---|---|---|---|---|---|---|
| CAS | |||||||
| Hislop (2009) [19] (3 MDs) d | 5 | 23 (19–42) vs. 83 (57–208) * | 12 (10–14) vs. 13 (7–15) | 57 (52–70) vs. 119 (57–175) * | N/A | GRS: 5 (4–5) | 1 |
| Roguin (2009) [20] (1 MD) | 1 | N/A | 2.5 min <average | 55 cc <average | N/A | N/A | 1 |
| Willaert (2010) [21] (2 MDs) | 1 | 24.04 vs. 60.44 | 11.19 vs. 21.04 | 70 vs. 120 | 6 vs. 13 | Likert: 4 (2–5) | 1 |
| Willaert (2012) [22] (3 MD) e | 15 | 23.75 (13–29) vs. 40.00 (28–62) *,^ | 9.70 (4.5–15) vs. 11.24 (7.5–22) ^ | 90.00 vs. 100.00 | 7 vs. 10 * | Likert: 4 | 1 |
| EVAR | |||||||
| Desender (2013) [23] (9 MDs) f | 10 ** | 32 (IQR 24–41) vs. 43 (IQR 39–60) * | 13 (IQR 11–16) vs. 10 (IQR 8–18) | 80 (IQR 75–97) vs. 80 (IQR 61–92) | 5 (IQR 4–8.5) vs. 6 (IQR 4.5–7) | Likert: 4 (IQR 3–5) | 1 |
| Pakeliani (2019) [24] (1 MD) g | 10 | 54 ± 14 (37–80) vs. 69 ± 16 (45–90) + | N/A | N/A | N/A | N/A | 1 |
| TEVAR | |||||||
| Desender (2017) [25] (2 MDs) | 2 | Sim. time for 1 patient: 16 | N/A | N/A | N/A | N/A | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, C.A.-B.; Lönn, L.; Konge, L.; Taudorf, M. Simulation-Based Virtual-Reality Patient-Specific Rehearsal Prior to Endovascular Procedures: A Systematic Review. Diagnostics 2020, 10, 500. https://doi.org/10.3390/diagnostics10070500
Nielsen CA-B, Lönn L, Konge L, Taudorf M. Simulation-Based Virtual-Reality Patient-Specific Rehearsal Prior to Endovascular Procedures: A Systematic Review. Diagnostics. 2020; 10(7):500. https://doi.org/10.3390/diagnostics10070500
Chicago/Turabian StyleNielsen, Caroline Albrecht-Beste, Lars Lönn, Lars Konge, and Mikkel Taudorf. 2020. "Simulation-Based Virtual-Reality Patient-Specific Rehearsal Prior to Endovascular Procedures: A Systematic Review" Diagnostics 10, no. 7: 500. https://doi.org/10.3390/diagnostics10070500
APA StyleNielsen, C. A.-B., Lönn, L., Konge, L., & Taudorf, M. (2020). Simulation-Based Virtual-Reality Patient-Specific Rehearsal Prior to Endovascular Procedures: A Systematic Review. Diagnostics, 10(7), 500. https://doi.org/10.3390/diagnostics10070500





