A Fluorescence In Situ Hybridization (FISH) Test for Diagnosing Babesiosis
Abstract
1. Introduction
2. Materials and Methods
2.1. FISH Assay Reagents
2.2. FISH Test Method
2.3. Analytical Specificity of the Babesia Genus FISH Test
2.4. Detection of Different Babesia Species in the Babesia Genus-Specific FISH Test
2.5. Analytical Sensitivity of the Babesia Genus FISH Test
2.6. Detection of B. duncani and B. microti in Clinical Blood Samples
2.7. Estimation of Clinical Diagnostic Parameters of the Babesia Genus FISH Test
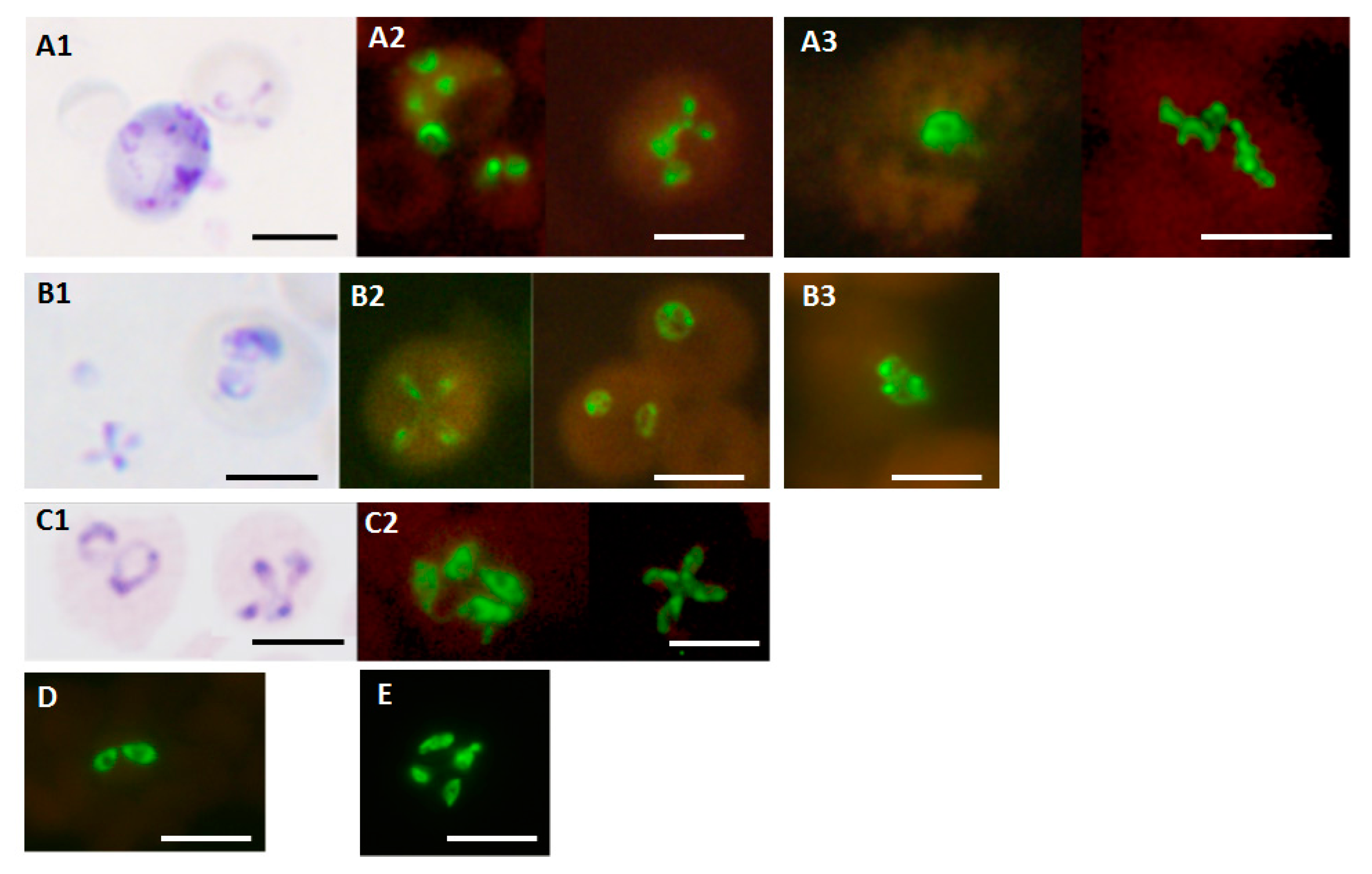
3. Results
3.1. Analytical Specificity
3.2. Detection of Different Babesia Species
3.3. Analytical Sensitivity
3.4. Detection of B. duncani and B. microti in Clinical Blood Samples
3.5. Estimated Clinical Diagnostic Parameters of the Babesis Genus FISH Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethics Statement
Disclaimer
References
- Vannier, E.; Gewurz, B.E.; Krause, P.J. Human babesiosis. Infect. Dis. Clin. N. Am. 2008, 22, 469–488. [Google Scholar] [CrossRef]
- Ord, R.L.; Lobo, C.A. Human babesiosis: Pathogens, prevalence, diagnosis and treatment. Curr. Clin. Microbiol. Rep. 2015, 2, 173–181. [Google Scholar] [CrossRef]
- Krause, P.J. Human babesiosis. Int. J. Parasitol. 2019, 49, 165–174. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Parasites—Babesiosis. 2020. Available online: https://www.cdc.gov/parasites/babesiosis/index.html (accessed on 7 May 2020).
- Brennan, M.B.; Herwaldt, B.L.; Kazmierczak, J.J.; Weiss, J.W.; Klein, C.L.; Leith, C.P.; He, R.; Oberley, M.J.; Tonnetti, L.; Wilkins, P.P.; et al. Transmission of Babesia microti parasites by solid organ transplantation. Emerg. Infect. Dis. 2016, 22, 1869–1876. [Google Scholar] [CrossRef]
- Conrad, P.A.; Kjemtrup, A.; Carreno, R.A.; Thomford, J.; Wainwright, K.; Eberhard, M.; Quick, R.; Telford, S.R., III; Herwaldt, B.L. Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int. J. Parasitol. 2006, 36, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; O’Connor, K.E.; Couper, L.I.; Thekkiniath, J.; Conrad, P.A.; Padgett, K.A.; Burns, J.; Yoshimizu, M.H.; Gonzales, B.; Munk, B.; et al. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int. J. Parasitol. 2019, 49, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Scott, C.M. Human babesiosis caused by Babesia duncani has widespread distribution across Canada. Healthcare 2018, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Mark, O.; Weltman, H.; Barcelo, N.; Lo, W.; Wronska, D.; Kakkilaya, S.; Rao, A.; Bhat, S.T.; Sinha, R.; et al. Fluorescence in situ hybridization (FISH) assays for diagnosing malaria in endemic areas. PLoS ONE 2015, 10, e0136726. [Google Scholar]
- Shah, J.; Poruri, A.; Mark, O.; Khadilka, U.; Mohring, F.; Moon, R.W.; Ramasamy, R. A dual colour fluorescence in situ hybridization (FISH) assay for identifying the zoonotic malaria parasite Plasmodium knowlesi with a potential application for the specific diagnosis of knowlesi malaria in peripheral-level laboratories of Southeast Asia. Parasites Vectors 2017, 10, 342. [Google Scholar] [CrossRef]
- Shah, J.; Weltman, H.; Narciso, P.; Murphy, C.; Poruri, A.; Baliga, S.; Sharon, L.; York, M.; Cunningham, G.; Miller, S.; et al. Dual color fluorescence in situ hybridization (FISH) assays for detecting Mycobacterium tuberculosis and Mycobacterium avium complexes and related pathogens in cultures. PLoS ONE 2017, 12, e0174989. [Google Scholar] [CrossRef]
- Baliga, S.; Murphy, C.; Sharon, L.; Shenoy, S.; Biranthabail, D.; Weltman, H.; Miller, S.; Ramasamy, R.; Shah, J. Rapid method for detecting and differentiating Mycobacterium tuberculosis complex and non-tuberculous mycobacteria in sputum by fluorescence in situ hybridization with DNA probes. Int. J. Infect. Dis. 2018, 75, 1–7. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Tick-Borne Relapsing Fever (TBRF). 2015. Available online: https://www.cdc.gov/relapsing-fever/index.html (accessed on 2 April 2020).
- Centers for Disease Control and Prevention. Lyme Disease. 2015. Available online: https://www.cdc.gov/lyme/index.html (accessed on 2 April 2020).
- Dunn, J.M.; Krause, P.J.; Davis, S.; Vannier, E.G.; Fitzpatrick, M.C.; Rollend, L.; Belperron, A.A.; States, S.L.; Stacey, A.; Bockenstedt, L.K.; et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS ONE 2014, 9, e115494. [Google Scholar] [CrossRef] [PubMed]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co-Infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. Trends. Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Djokic, V.; Primus, S.; Akoolo, L.; Chakraborti, M.; Parveen, N. Age-related differential stimulation of immune response by Babesia microti and Borrelia burgdorferi during acute phase of infection affects disease severity. Front. Immunol. 2018, 9, 2891. [Google Scholar] [CrossRef] [PubMed]
- Primus, S.; Akoolo, L.; Schlachter, S.; Gedroic, K.; Rojtman, A.D.; Parveen, N. Efficient detection of symptomatic and asymptomatic patient samples for Babesia microti and Borrelia burgdorferi infection by multiplex qPCR. PLoS ONE 2018, 13, e0196748. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R. Molecular basis for immune evasion and pathogenesis in malaria. Biochim. Biophys. Acta 1998, 1406, 10–27. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Rojas, C.; Figueroa, J.V. Diagnostic tools for the identification of Babesia sp. in persistently infected cattle. Pathogens 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.E.; Thomas, J.E.; Wohltjen, M.L.; Reichard, M.V. Transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum nymphs. Parasites Vectors 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Villafuerte, P.; Zhuge, J.; Visintainer, P.; Wormser, G.P. Comparison of a quantitative PCR assay with peripheral blood smear examination for detection and quantitation of Babesia microti infection in humans. Diagn. Microbiol. Infect. Dis. 2015, 82, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Akoolo, L.; Schlachter, S.; Khan, R.; Alter, L.; Rojtman, A.D.; Gedroic, K.; Bhanot, P.; Parveen, N. A novel quantitative PCR detects Babesia infection in patients not identified by currently available non-nucleic acid amplification tests. BMC Microbiol. 2017, 17, 16. [Google Scholar] [CrossRef]
- Souza, S.S.; Bishop, H.S.; Sprinkle, P.; Qvarnstrom, Y. Comparison of Babesia microti real-time polymerase chain reaction assays for confirmatory diagnosis of babesiosis. Am. J. Trop. Med. Hyg. 2016, 95, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.J.; Stramer, S.L.; Szczepiorkowski, Z.M. Assessing the risk of Babesia to the United States blood supply using a risk-based decision-making approach: Report of AABB’s ad hoc Babesia policy working group. Transfusion 2018, 58, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Vesey, G.; Ashbolt, N.; Fricker, E.J.; Deere, D.; Williams, K.L.; Veal, D.A.; Dorsch, M. The use of a ribosomal RNA targeted oligonucleotide probe for fluorescent labelling of viable Cryptosporidium parvum oocysts. J. Appl. Microbiol. 1998, 85, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Cruz, I.D.; Ward, S.; Harris, N.S.; Ramasamy, R. Development of a sensitive PCR-dot blot assay to supplement serological tests for diagnosing Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 37, 701–709. [Google Scholar] [CrossRef]
- DeLong, E.F.; Wickham, G.S.; Pace, N.R. Phylogenetic stains: Ribosomal RNA-based probes for the identification of single cells. Science 1989, 1243, 1360–1363. [Google Scholar] [CrossRef]
- Jiang, J.F.; Zheng, Y.C.; Jiang, R.R.; Li, H.; Huo, Q.B.; Jiang, B.G.; Sun, Y.; Jia, N.; Wang, Y.W.; Ma, L.; et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: A descriptive study. Lancet Infect. Dis. 2015, 15, 196–203. [Google Scholar] [CrossRef]
- Shah, J.S.; Horowitz, R.; Harris, N.S. Human babesiosis and ehrlichiosis—Current status. Europ. Infect. Dis. 2012, 6, 49–56. [Google Scholar]
| Bacteria | Source |
|---|---|
| Anaplasma phagocytophilum | Patient blood from IGeneX positive for Anaplasma phagocytophilum |
| Bartonella henselae | ATCC 49882 in vitro culture |
| Borrelia burgdorferi | ATCC 35210-B31 in vitro culture |
| Borrelia hermsii | DSM 5251 in vitro culture |
| Ehrlichia chaffeensis | Patient blood from IGeneX positive for Ehrlichia chaffeensis |
| Protozoa | |
| Leishmania donovani | ATCC 50212 in vitro culture |
| Plasmodium falciparum | Patient blood from Kenya |
| Plasmodium malariae | Patient blood from Kenya |
| Plasmodium ovale | Patient blood from Kenya |
| Plasmodium vivax | Patient blood from India |
| Theileria equi | BEG-120 in vitro culture in equine blood, Fuller Lab, Fullerton, CA |
| Trypanosoma cruzi | ATCC 50823 in vitro culture |
| Controls | |
| Negative control | Uninfected human blood |
| Positive control 1 | Hamster blood infected with Babesia duncani ATCC PRA-302 |
| Positive control 2 | Hamster blood infected with Babesia microti ATCC 30221D |
| FISH | Giemsa | Clinical Diagnostic Parameter | Estimate (95% CI) | |
|---|---|---|---|---|
| (+) | (−) | Sensitivity | 98% (88–100) | |
| (+) | 50 | 0 | Specificity | 100% (96–100) |
| (−) | 1 | 103 | Positive Predictive Value | 100% (91–100) |
| Total | 51 | 103 | Negative Predictive Value | 99% (94–100) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, J.S.; Mark, O.; Caoili, E.; Poruri, A.; Horowitz, R.I.; Ashbaugh, A.D.; Ramasamy, R. A Fluorescence In Situ Hybridization (FISH) Test for Diagnosing Babesiosis. Diagnostics 2020, 10, 377. https://doi.org/10.3390/diagnostics10060377
Shah JS, Mark O, Caoili E, Poruri A, Horowitz RI, Ashbaugh AD, Ramasamy R. A Fluorescence In Situ Hybridization (FISH) Test for Diagnosing Babesiosis. Diagnostics. 2020; 10(6):377. https://doi.org/10.3390/diagnostics10060377
Chicago/Turabian StyleShah, Jyotsna S., Olivia Mark, Eddie Caoili, Akhila Poruri, Richard I. Horowitz, Alan D. Ashbaugh, and Ranjan Ramasamy. 2020. "A Fluorescence In Situ Hybridization (FISH) Test for Diagnosing Babesiosis" Diagnostics 10, no. 6: 377. https://doi.org/10.3390/diagnostics10060377
APA StyleShah, J. S., Mark, O., Caoili, E., Poruri, A., Horowitz, R. I., Ashbaugh, A. D., & Ramasamy, R. (2020). A Fluorescence In Situ Hybridization (FISH) Test for Diagnosing Babesiosis. Diagnostics, 10(6), 377. https://doi.org/10.3390/diagnostics10060377






