Development of Stiffness Measurement Program Using Color Mapping in Shear Wave Elastography
Abstract
1. Introduction
2. Materials and Methods
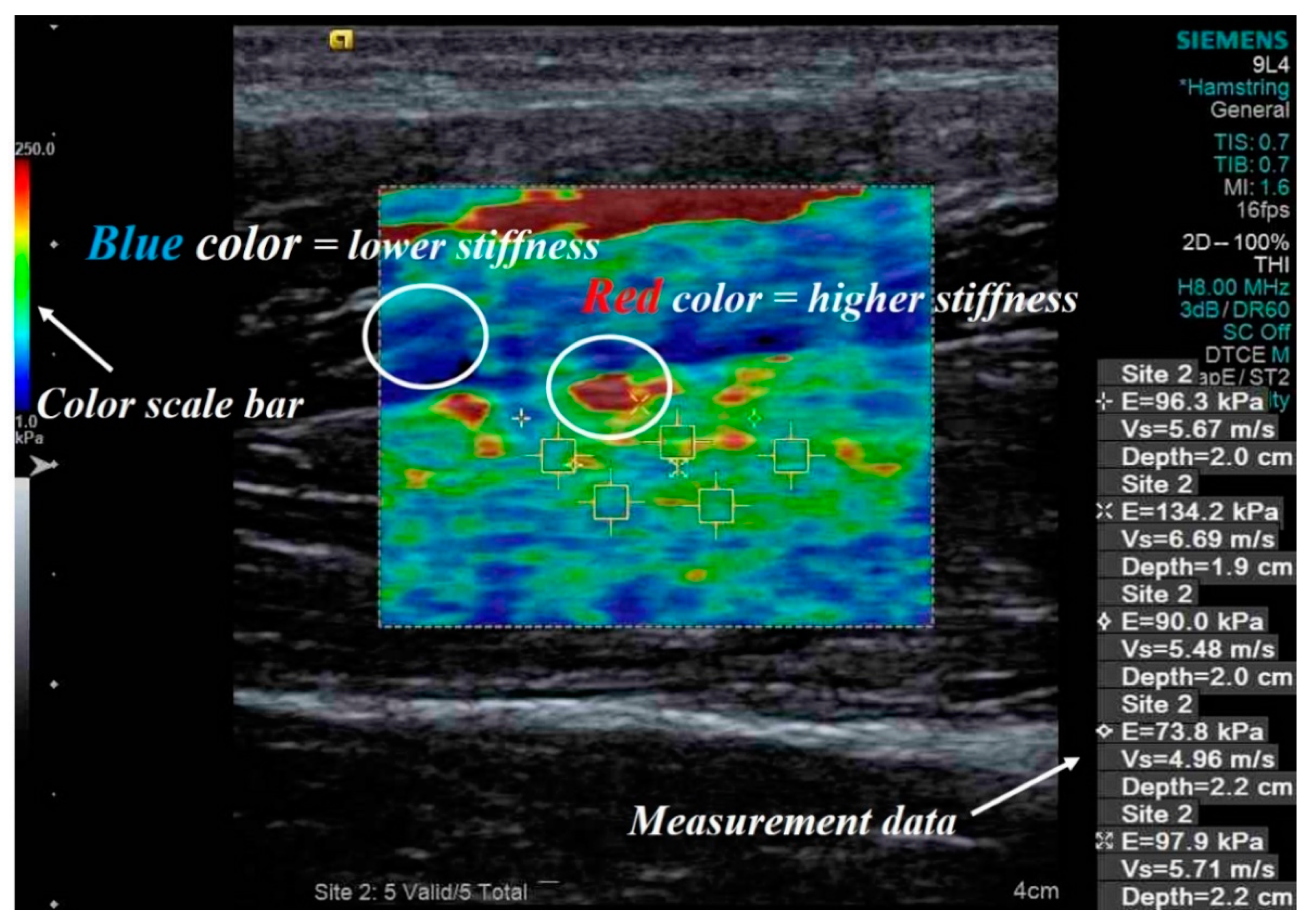
2.1. Used Shear Wave Elastography Ultrasound
2.2. Procedure
2.3. Used Shear Wave Elastography Ultrasound
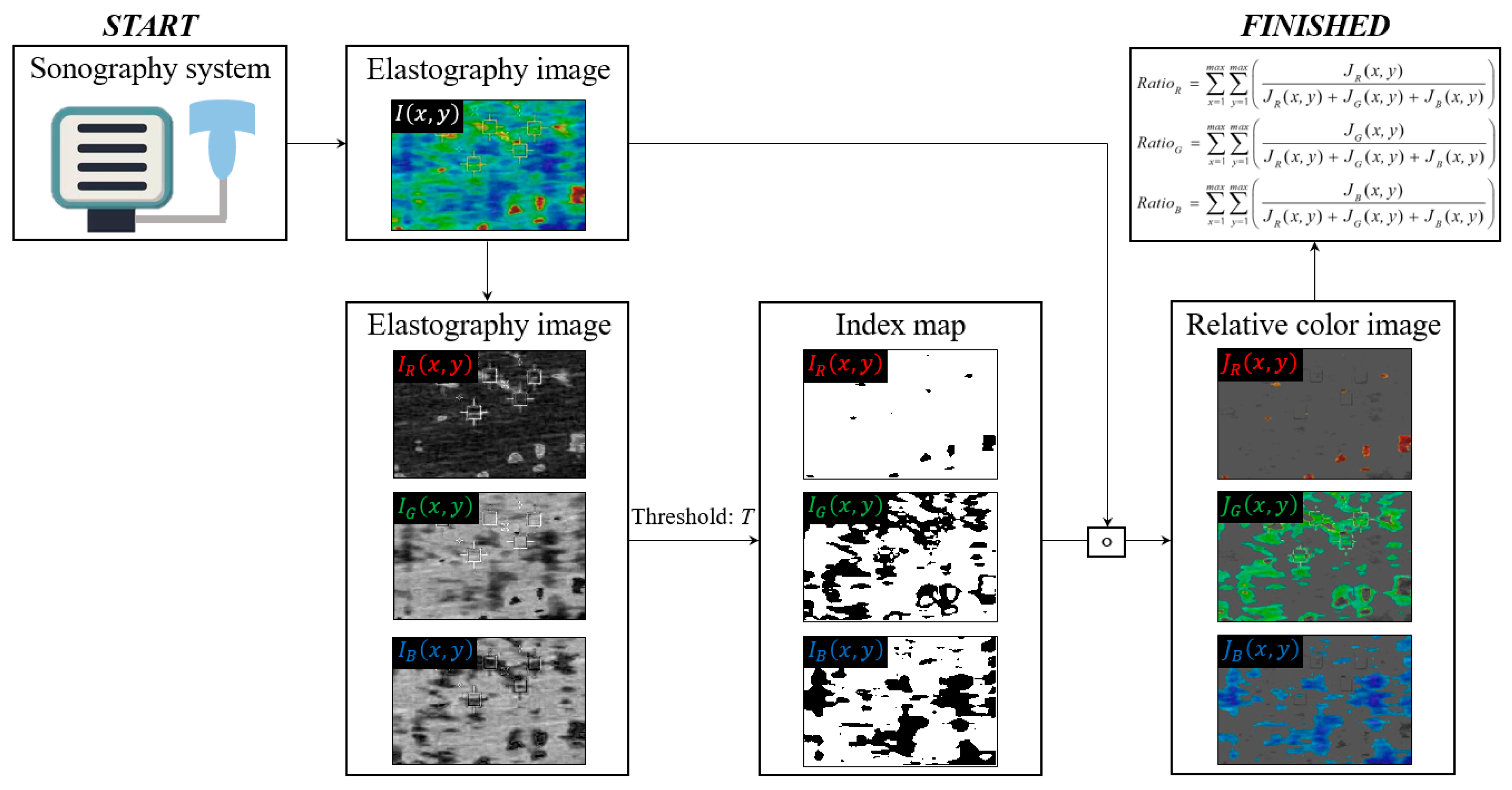
2.4. Stiffness Measurement Program Using Color Mapping
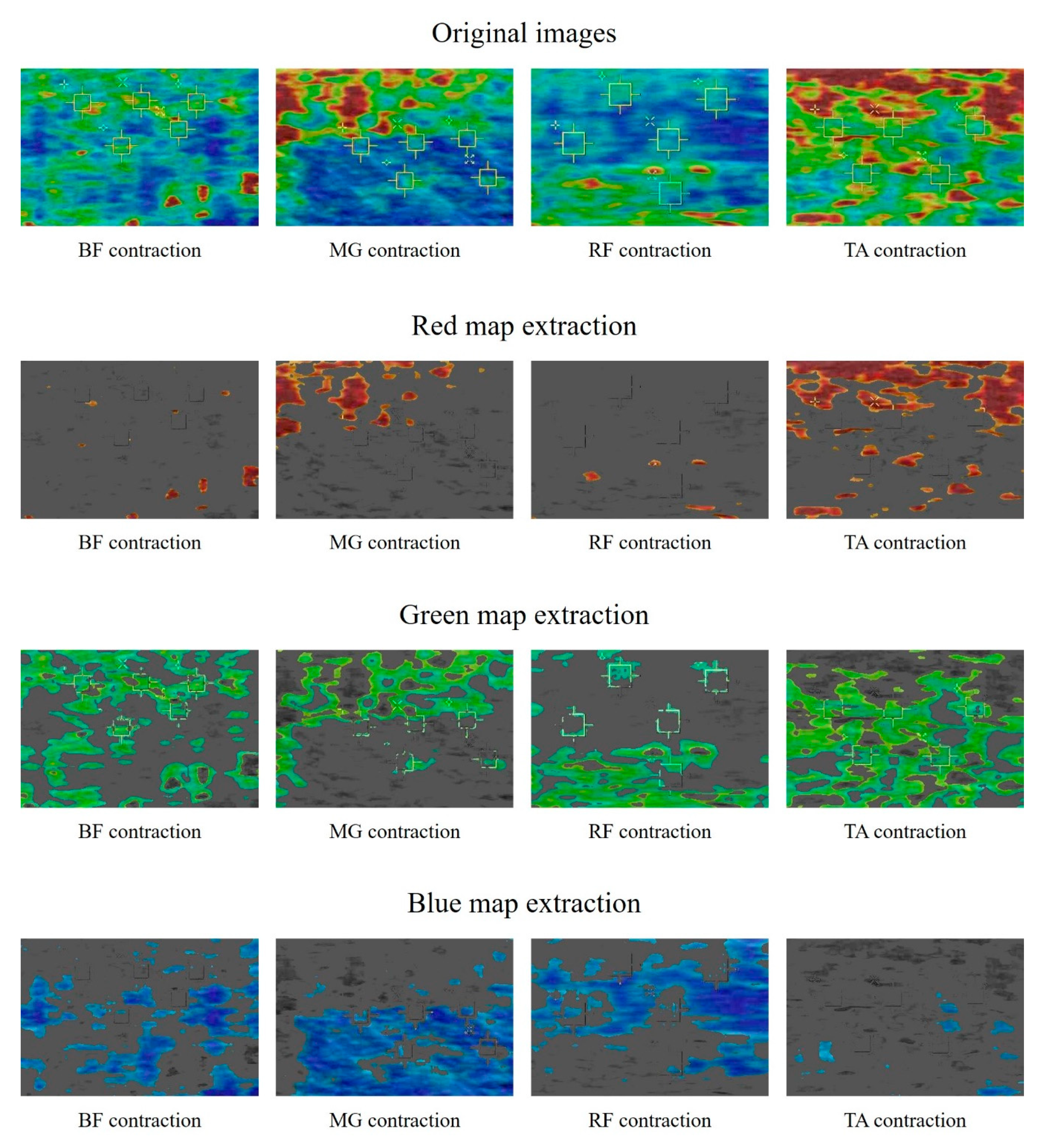
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brenner, D.J.; Hall, E.J.; Phil, D. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.J.; Costa, H.S.; Silva, P.D.; Anderson, J.L.; Arshad, A.; Biederman, R.W.W.; Boyle, N.G.; Frabizzio, J.V.; Birgersdotter-Green, U.; Higgins, S.L.; et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N. Engl. J. Med. 2017, 376, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kim, Y.S. Ultrasound imaging and beyond: Recent advances in medical ultrasound. Biomed. Eng. Lett. 2017, 7, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean society of thyroid radiology consensus statement and recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef]
- Schaub, B.; Gueneret, M.; Jolivet, E.; Decatrelle, V.; Yazza, S.; Gueye, H.; Monthieux, A.; Juve, M.L.; Gautier, M.; Najioullah, F.; et al. Ultrasound imaging for identification of cerebral damage in congenital Zika virus syndrome: A case series. Lancet Child Adolesc. Health 2017, 1, 45–55. [Google Scholar] [CrossRef]
- Maturen, K.E.; Wasnik, A.P.; Kamaya, A.; Dillman, J.R.; Kaza, R.K.; Pandya, A.; Maheshwary, R.K. Ultrasound imaging of bowel pathology: Technique and keys to diagnosis in the actute abdomen. Am. J. Roentgenol. 2011, 197, W1067–W1075. [Google Scholar] [CrossRef]
- Witvrouw, E.; Mahieu, N.; Roosen, P.; McNair, P. The role of stretching in tendon injuries. Br. J. Sports Med. 2007, 41, 224–226. [Google Scholar] [CrossRef]
- Lee, S.H.; Chang, J.M.; Chon, N.; Koo, H.R.; Yi, A.; Kim, S.J.; Youk, J.H.; Son, E.J.; Choi, S.H.; Kook, S.H.; et al. Practice guideline for the performance of breast ultrasound elastography. Ultrasonography 2014, 33, 3–10. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and technique. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Kelly, J.P.; Koppenhaver, S.L.; Michener, L.A.; Proulx, L.; Bisagni, F.; Cleland, J.A. Characterization of tissue stiffness of the infraspinatus, erector spinae, and gastrocnemius muscle using ultrasound shear wave elastography and superficial mechanical deformation. J. Elctromyography Kinesiol. 2018, 38, 73–80. [Google Scholar] [CrossRef]
- Prado-Costa, R.; Rebelo, J.; Monteiro-Barroso, J.; Preto, A.S. Ultrasound elastography: Compression elastography and shear-wave elastography in the assessment of tendon injury. Insights Imaging 2018, 9, 791–814. [Google Scholar] [CrossRef]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, C.; Larsen, A.H.; Hartvigsen, J. A systematic, critical review of manual palpation for identifying myofascial trigger points: Evidence and clinical significance. Arch. Phys. Med. Rehabil. 2008, 89, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, J.; Gotugno, G.; Dasgupta, P.; Althoefer, K.; Nanayakkara, T. Palpation force modulation strategies to identify hard regions in soft tissue organs. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Chaves, T.C.; Nagamine, H.M.; de Sousa, L.M.; de Oliveira, A.S.; Grossi, D.B. Comparison between the reliability levels of manual palpation and pressure patin threshold in children who reported orofacial pain. Man. Ther. 2010, 15, 508–512. [Google Scholar] [CrossRef]
- Alam, F.; Naito, K.; Horiguchi, J.; Fukuda, H.; Tachikake, T.; Ito, K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparision with conventional B-mode sonography. Am. J. Roentgenol. 2008, 191, 604–610. [Google Scholar] [CrossRef]
- Dudea, S.M.; Botar-Jid, C.; Dumitrium, D.; Vasilescu, D.; MAnole, S.; Lenghel, M.L. Differentiaing benign from malignant superficial lymph nodes with sonoelastography. Med. Ultrason. 2013, 15, 132–139. [Google Scholar] [CrossRef][Green Version]
- Evans, A.; Whelehan, P.; Thomson, K.; McLean, D.; Brauer, K.; Purdie, C.; Baker, L.; Jordan, L.; Rauchhaus, P.; Thompson, A. Invasive breast cancer: Relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology 2012, 263, 673–677. [Google Scholar] [CrossRef]
- Ham, S.; Kim, S.; Choi, H.; Lee, Y.; Lee, H. Greater muscle stiffness during contraction at menstruation as measured by shear-wave elastography. Tohoku J. Exp. Med. 2020, 250, 207–213. [Google Scholar] [CrossRef]
- Lee, S.S.M.; Gaebler-Spira, D.; Zhang, L.Q.; Rymer, W.Z.; Steele, K.M. Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin. Biomechnics 2016, 31, 20–28. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver ultrasound elastography: An update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.S.S.; Cho, C.C.M.; Yuen, Y.H.; Rasalkar, D.D.; King, A.D.; Ahuja, A.T. Real-time qualitative ultrasound elastography of cervical lymph nodes in routine clinical practice: Interobserver agreement and correlation with malignancy. Ultrasound Med. Biol. 2010, 36, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, J.Y.; Yoo, Y. A New Feature-Enhanced Speckle Reduction Method Based on Multiscale Analysis for Ultrasound B-Mode Imaging. IEEE Trans. Biomed. Eng. 2016, 63, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Dantas, R.G.; Costa, E.T.; Leeman, S. Ultrasound speckle and equivalent scatterers. Ultrasonics 2005, 43, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Tay, P.C.; Acton, S.T.; Hossack, J.A. A Wavelet Thresholding Method to Reduce Ultrasound Artifacts. Comput. Med. Imaging Graph. 2011, 35, 42–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, S.H.; Kim, M.; Lee, Y. The study on reduction for near field clutter (NFC) artifact based on wavelet thresholding method in ultrasound image using Field II program. Optik 2018, 162, 220–227. [Google Scholar] [CrossRef]

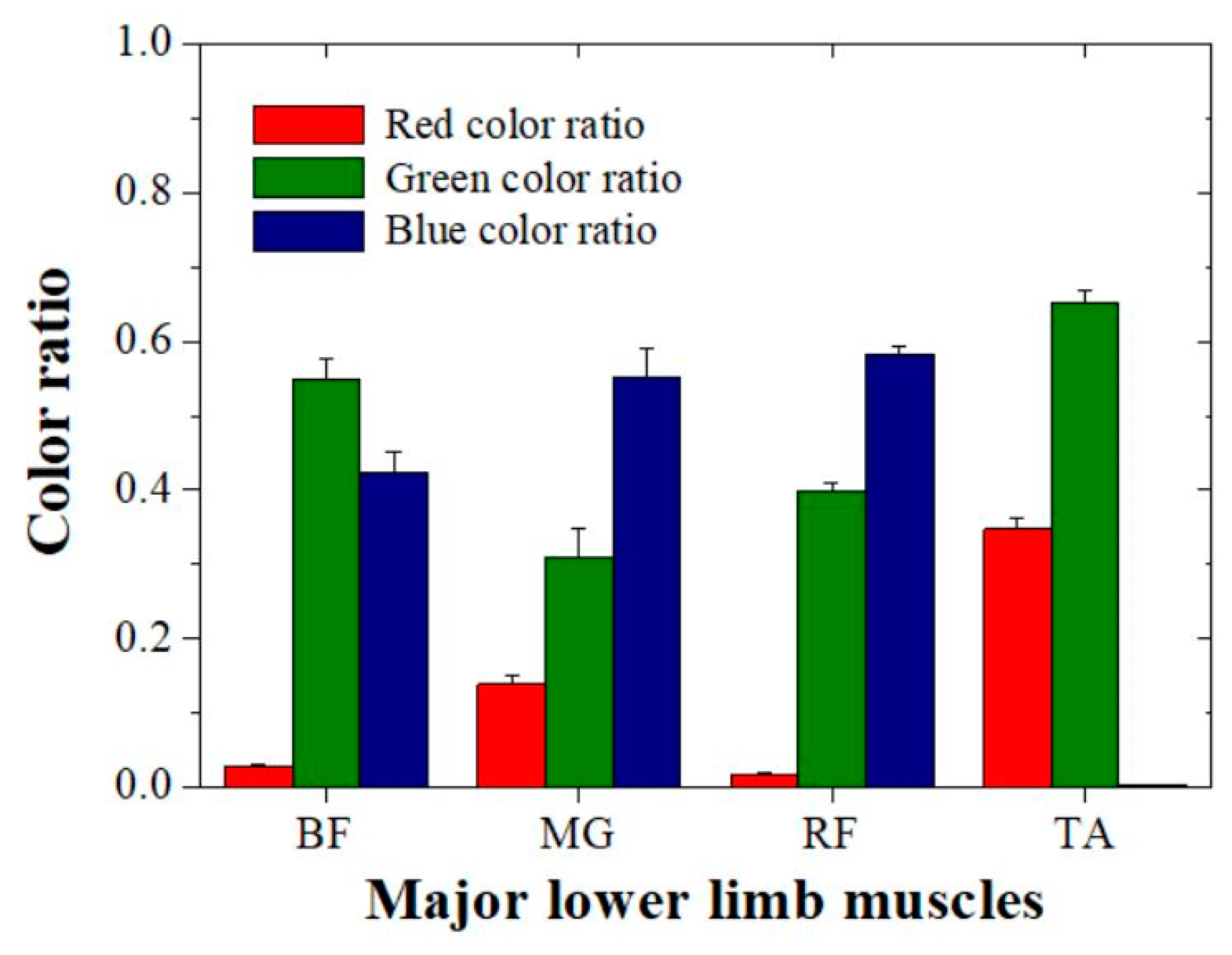
| Muscle | Color Ratio Data | |
|---|---|---|
| Red Color | Blue Color | |
| BF | 0.028 ± 0.0023 | 0.423 ± 0.0265 |
| MG | 0.139 ± 0.0128 | 0.552 ± 0.0368 |
| RF | 0.018 ± 0.0015 | 0.583 ± 0.0105 |
| TA | 0.347 ± 0.0161 | 0.002 ± 0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, K.; Lee, Y. Development of Stiffness Measurement Program Using Color Mapping in Shear Wave Elastography. Diagnostics 2020, 10, 362. https://doi.org/10.3390/diagnostics10060362
Lee H, Kim K, Lee Y. Development of Stiffness Measurement Program Using Color Mapping in Shear Wave Elastography. Diagnostics. 2020; 10(6):362. https://doi.org/10.3390/diagnostics10060362
Chicago/Turabian StyleLee, Haneul, Kyuseok Kim, and Youngjin Lee. 2020. "Development of Stiffness Measurement Program Using Color Mapping in Shear Wave Elastography" Diagnostics 10, no. 6: 362. https://doi.org/10.3390/diagnostics10060362
APA StyleLee, H., Kim, K., & Lee, Y. (2020). Development of Stiffness Measurement Program Using Color Mapping in Shear Wave Elastography. Diagnostics, 10(6), 362. https://doi.org/10.3390/diagnostics10060362






