Diagnostic Value of Imaging Methods in the Histological Four Grading of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
- Child-Pugh grade C liver cirrhosis.
- Liver CEUS not performed within one month prior to the biopsy.
- No reliable or conclusive pathological diagnosis could be obtained because of insufficient biopsy specimens from the lesion, or a ‘‘no-hit’’ result for the target lesion during a percutaneous biopsy.
- Systemic chemotherapy or targeted treatment administered prior to the CEUS, which could potentially influence the findings on the CEUS images.
2.2. Grayscale US and Sonazoid CEUS Examination
2.3. Gd-EOB-DTPA MRI
2.4. Diagnostic Criteria
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparison of the Grayscale US Patterns, and the CEUS and Gd-EOB-DTPA MRI Enhancement Patterns among the Four Histological Grades
3.3. Analysis of the Diagnostic Efficacy of Each of the Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farinati, F.; Sergio, A.; Baldan, A.; Giacomin, A.; Di Nolfo, M.A.; Del Poggio, P.; Benvegnù, L.; Rapaccini, G.L.; Zoli, M.; Borzio, F.; et al. Early and very early hepatocellular carcinoma: When and how much do staging and choice of treatment really matter? A multi-center study. BMC Cancer 2009, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Takayama, T.; Higaki, T.; Watanabe, Y.; Makuuchi, M. Clinical significance of early hepatocellular carcinoma. Liver Transplant. 2004, 10, S16–S19. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Kato, T.; Berho, M.; Misiakos, E.P.; O’Brien, C.; Reddy, K.R.; Nery, J.R.; Burke, G.W.; Schiff, E.R.; Miller, J.; et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001, 136, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.E.; Kakar, S.; Mehta, N.; Gill, R.M. A Point-based Histologic Scoring System for Hepatocellular Carcinoma Can Stratify Risk of Posttransplant Tumor Recurrence. Am. J. Surg. Pathol. 2018, 42, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mao, J.; Li, W. Association of tumor grade with long-term survival in patients with hepatocellular carcinoma after liver transplantation. Transplant. Proc. 2019, 51, 813–819. [Google Scholar] [CrossRef]
- Takayama, T.; Makuuchi, M.; Hirohashi, S.; Sakamoto, M.; Yamamoto, J.; Shimada, K.; Kosuge, T.; Okada, S.; Takayasu, K.; Yamasaki, S. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology 1998, 28, 1241–1246. [Google Scholar] [CrossRef]
- Sasaki, K.; Matsuda, M.; Ohkura, Y.; Kawamura, Y.; Inoue, M.; Hashimoto, M.; Ikeda, K.; Kumada, H.; Watanabe, G. In hepatocellular carcinomas, any proportion of poorly differentiated components is associated with poor prognosis after hepatectomy. World J. Surg. 2013, 38, 1147–1153. [Google Scholar] [CrossRef]
- Han, C.; Gao, L.; Zhao, L.; Sheng, Q.; Zhang, C.; An, Z.; Xia, T.; Ding, Y.; Wang, J.; Bai, H.; et al. Immunohistochemistry detects increased expression of aldo-keto reductase family 1 member b10 (AKR1B10) in early-stage hepatocellular carcinoma. Med. Sci. Monit. 2018, 24, 7414–7423. [Google Scholar] [CrossRef]
- Jonas, S.; Bechstein, W.O.; Steinmüller, T.; Herrmann, M.; Radke, C.; Berg, T.; Settmacher, U.; Neuhaus, P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001, 33, 1080–1086. [Google Scholar] [CrossRef]
- Kondo, F. Histological features of early hepatocellular carcinomas and their developmental process: For daily practical clinical application. Hepatol. Int. 2008, 3, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Sciarra, A.; Di Tommaso, L.; Nakano, M.; Destro, A.; Torzilli, G.; Donadon, M.; Maggioni, M.; Bosari, S.; Bulfamante, G.; Matsuda, M.; et al. Morphophenotypic changes in human multistep hepatocarcinogenesis with translational implications. J. Hepatol. 2016, 64, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Working Party. Terminology of nodular hepatocellular lesions. Hepatology 1995, 22, 983–993. [Google Scholar] [CrossRef]
- International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: A report of the international consensus group for hepatocellular neoplasia. Hepatology 2008, 49, 658–664. [Google Scholar]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
- Martins-Filho, S.N.; Paiva, C.; Azevedo, R.S.; Alves, V.A.F. Histological Grading of Hepatocellular Carcinoma—A Systematic Review of Literature. Front. Med. 2017, 4, 193. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Song, Z.; Du, M.; Klibanov, A.L. Ultrasound molecular imaging for differentiation of benign and malignant tumors in patients. Quant. Imaging Med. Surg. 2018, 8, 1078–1083. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, S.Y.; Tang, A.; Yoon, J.H. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin. Mol. Hepatol. 2019, 25, 245–263. [Google Scholar] [CrossRef] [Green Version]
- Nevin, Y.; Yilmaz, U.E.; Suer, K.; Goral, V.; Cakir, N. Screening for hepatocellular carcinoma: Summary of current guidelines up to 2018. Hepatoma Res. 2018, 46, 1–10. [Google Scholar]
- Jiang, H.-Y.; Chen, J.; Xia, C.-C.; Cao, L.-K.; Duan, T.; Song, B. Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J. Gastroenterol. 2018, 24, 2348–2362. [Google Scholar] [CrossRef]
- Kim, H.Y.; Choi, J.Y.; Kim, C.W.; Bae, S.H.; Yoon, S.K.; Lee, Y.J.; Rha, S.E.; You, Y.K.; Kim, D.G.; Jung, E.S. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging predicts the histological grade of hepatocellular carcinoma only in patients with child-pugh class a cirrhosis. Liver Transplant. 2012, 18, 850–857. [Google Scholar] [CrossRef]
- Arita, J.; Hasegawa, K.; Takahashi, M.; Hata, S.; Shindoh, J.; Sugawara, Y.; Kokudo, N. Correlation between contrast-enhanced intraoperative ultrasound using sonazoid and histologic grade of resected hepatocellular carcinoma. Am. J. Roentgenol. 2011, 196, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Fukuda, H.; Nihonmatsu, H.; Kondo, M.; Nozaki, A.; Chuma, M.; Morimoto, M.; Oshima, T.; Okada, M.; Murakami, T.; et al. Use of vessel patterns on contrast-enhanced ultrasonography using a perflubutane-based contrast agent for the differential diagnosis of regenerative nodules from early hepatocellular carcinoma or high-grade dysplastic nodules in patients with chronic liver disease. Abdom. Imaging 2015, 40, 2372–2383. [Google Scholar] [PubMed]
- Duisyenbi, Z.; Numata, K.; Nihonmatsu, H.; Fukuda, H.; Chuma, M.; Kondo, M.; Nozaki, A.; Tanaka, K.; Maeda, S. Comparison between low mechanical index and high mechanical index contrast modes of contrast-enhanced ultrasonography: Evaluation of perfusion defects of hypervascular hepatocellular carcinomas during the post-vascular phase. J. Ultrasound Med. 2019, 38, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Saito, A.; Yamamoto, M.; Doi, M.; Takasaki, K. Stromal and blood vessel wall invasion in well-differentiated hepatocellular carcinoma. Liver 1997, 17, 41–46. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kim, S.R.; Imoto, S.; Ando, K.; Hirakawa, M.; Saito, J.; Fukuda, K.; Otono, Y.; Sakaki, M.; Tsuchida, S.; et al. Histopathological Diagnosis of Early HCC through Biopsy: Efficacy of Victoria Blue and Cytokeratin 7 Staining. Dig. Dis. 2012, 30, 574–579. [Google Scholar] [CrossRef]
- Park, Y.N.; Kojiro, M.; Di Tommaso, L.; Dhillon, A.P.; Kondo, F.; Nakano, M.; Sakamoto, M.; Theise, N.D.; Roncalli, M. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer 2007, 109, 915–923. [Google Scholar] [CrossRef]
- Maeda, T.; Adachi, E.; Kajiyama, K.; Takenaka, K.; Honda, H.; Sugimachi, K.; Tsuneyoshi, M. CD34 expression in endothelial cells of small hepatocellular carcinoma: Its correlation with tumour progression and angiographic findings. J. Gastroenterol. Hepatol. 1995, 10, 650–654. [Google Scholar] [CrossRef]
- Tummala, K.S.; Brandt, M.; Teijeiro, A.; Graña, O.; Schwabe, R.F.; Perna, C.; Djouder, N. Hepatocellular carcinomas originate predominantly from hepatocytes and benign lesions from hepatic progenitor cells. Cell Rep. 2017, 19, 584–600. [Google Scholar] [CrossRef] [Green Version]
- Barakauskienė, A.; Speičienė, D.; Liakina, V.; Semuchinienė, T.; Valantinas, J. Expression of cytokeratin 7 as a histological marker of cholestasis and stages of primary biliary cirrhosis. Medicina (Kaunas) 2011, 47, 5. [Google Scholar] [CrossRef]
- Minami, Y.; Kudo, M. Hepatic malignancies: Correlation between sonographic findings and pathological features. World J. Radiol. 2010, 2, 249–256. [Google Scholar] [CrossRef]
- Nowicki, T.K.; Markiet, K.; Szurowska, E. Diagnostic imaging of hepatocellular carcinoma—A pictorial essay. Curr. Med. Imaging Rev. 2017, 13, 140–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannella, R.; Furlan, A. Mosaic architecture of hepatocellular carcinoma. Abdom. Radiol. 2017, 43, 1847–1848. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Matsui, O.; Ueda, K.; Kawamori, Y.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Takashima, T.; Nonomura, A.; Nakanuma, Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: Evaluation by CT during intraarterial injection of contrast medium. Am. J. Roentgenol. 1999, 172, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitao, A.; Zen, Y.; Matsui, O.; Gabata, T.; Nakanuma, Y. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography—Radiologic-pathologic correlation 1. Radiology 2009, 252, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Fukuda, H.; Miwa, H.; Ishii, T.; Moriya, S.; Kondo, M.; Nozaki, A.; Morimoto, M.; Okada, M.; Takebayashi, S.; et al. Contrast-enhanced ultrasonography findings using a perflubutane-based contrast agent in patients with early hepatocellular carcinoma. Eur. J. Radiol. 2014, 83, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, R.; Zhang, X.-H.; Tang, C.-L.; Ma, K.-S.; Guo, D.-Y.; Yan, X.-C. Perfusion characteristics of hepatocellular carcinoma at contrast-enhanced ultrasound: influence of the cellular differentiation, the tumor size and the underlying hepatic condition. Sci. Rep. 2018, 8, 4713. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.-J.; Kim, T.K.; Burns, P.N.; Wilson, S.R. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: Comparison with histologic differentiation. Radiology 2007, 244, 898–906. [Google Scholar] [CrossRef]
- Tanaka, H.; Iijima, H.; Higashiura, A.; Yoh, K.; Ishii, A.; Takashima, T.; Sakai, Y.; Aizawa, N.; Iwata, K.; Ikeda, N.; et al. New malignant grading system for hepatocellular carcinoma using the Sonazoid contrast agent for ultrasonography. J. Gastroenterol. 2013, 49, 755–763. [Google Scholar] [CrossRef]
- Imai, Y.; Murakami, T.; Yoshida, S.; Nishikawa, M.; Ohsawa, M.; Tokunaga, K.; Murata, M.; Shibata, K.; Zushi, S.; Kurokawa, M.; et al. Superparamagnetic iron oxide–enhanced magnetic resonance images of hepatocellular carcinoma: Correlation with histological grading. Hepatology 2000, 32, 205–212. [Google Scholar] [CrossRef]
- Haimerl, M.; Verloh, N.; Zeman, F.; Fellner, C.; Nickel, D.; Lang, S.A.; Teufel, A.; Stroszczynski, C.; Wiggermann, P. Gd-EOB-DTPA-enhanced MRI for evaluation of liver function: Comparison between signal-intensity-based indices and T1 relaxometry. Sci. Rep. 2017, 7, 43347. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, M.; Cai, H.; Chan, T.; Dong, Z.; Luo, Y.; Li, Z.-P.; Feng, S.-T. Gd-EOB-DTPA-enhanced magnetic resonance imaging combined with T1 mapping predicts the degree of differentiation in hepatocellular carcinoma. BMC Cancer 2016, 16, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Kim, S.H.; Park, M.J.; Park, C.K.; Rhim, H. Gadoxetic acid–enhanced hepatobiliary phase MRI and High-b-Value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. Am. J. Roentgenol. 2011, 197, W868–W875. [Google Scholar] [CrossRef] [PubMed]
- Mita, K.; Kim, S.R.; Kudo, M.; Imoto, S.; Nakajima, T.; Ando, K.; Fukuda, K.; Matsuoka, T.; Maekawa, Y.; Hayashi, Y. Diagnostic sensitivity of imaging modalities for hepatocellular carcinoma smaller than 2 cm. World J. Gastroenterol. 2010, 16, 4187–4192. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, L.; Destro, A.; Seok, J.Y.; Balladore, E.; Terracciano, L.; SanGiovanni, A.; Iavarone, M.; Colombo, M.; Jang, J.J.; Yu, E.; et al. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J. Hepatol. 2009, 50, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Numata, K.; Nihonmatsu, H.; Okada, M.; Maeda, S. Application of new ultrasound techniques for focal liver lesions. J. Med. Ultrason. 2020, 47, 215–237. [Google Scholar] [CrossRef] [PubMed]

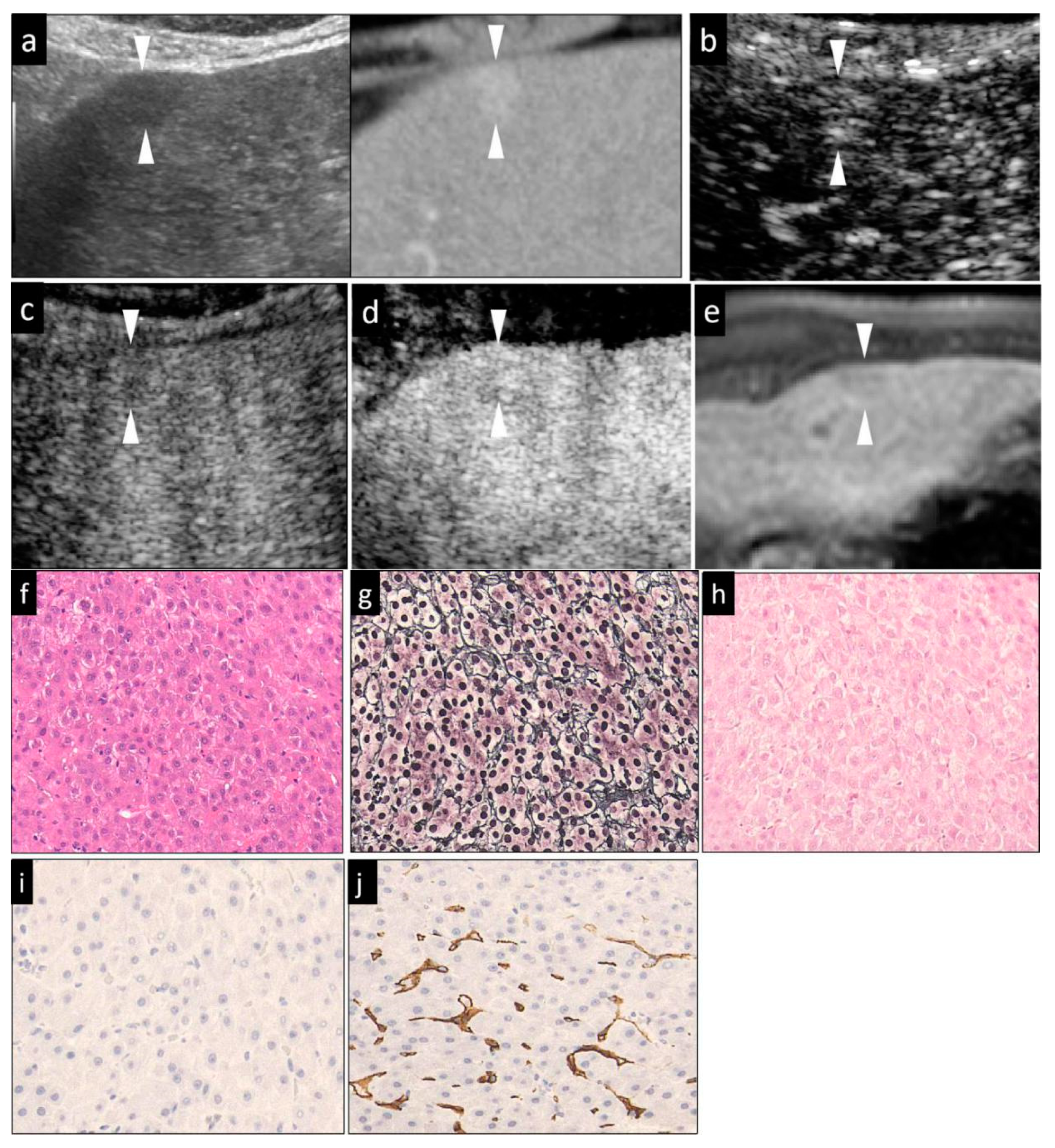


| Estimated Item | Cellular Atypia | Structural Atypia | Reticular Fibers | Neovascularization | Stromal Invasion | Ductular Reaction | ||
|---|---|---|---|---|---|---|---|---|
| Stain Method | HE | HE | Silver | CD34 2 | VB 3 | CK7 4 | ||
| Histological differentiation grade | Early HCC | Slightly enlarged, non-spherical nuclei; Mild hyper-cellularity; slight increase of the N/C ratio | Normal or clear thin trabeculae | Clear and evenly distributed | Negative or focal | Presence | Decrease or absence | |
| Advanced HCC | Well-differentiated | Large, irregularly shaped of nucleus; hypercellularity; increased N/C ratio | Thick trabeculae | Clear and recognizable | Diffuse | Absence | Absence | |
| Moderately differentiated | Markedly enlarged, deformed nuclei; hypercellularity; increased N/C ration; multinucleated giant cells (occasionally seen) | Thick trabeculae or solid structures | Sometimes unclear and sparse | Diffuse | Absence | Absence | ||
| Poorly differentiated | Large, bizarre-shaped nuclei; marked increase of the N/C ratio; multinucleated giant cells (frequently seen) | Disappeared trabecular and solid structure | Disappear | Diffuse | Absence | Absence | ||
| Histological Grade | Early HCC | Well-Differentiated HCC | Moderately Differentiated HCC | Poorly Differentiated HCC | p-Value 3 |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| No. of patients | 29 | 24 | 55 | 49 | / |
| Age (mean ± SD, years) | 70.33 ± 13.531 | 71.39 ± 8.036 | 70.07 ± 8.941 | 72.23 ± 10.248 | 0.493 |
| Sex (Female/male) | 10/35 | 6/25 | 19/49 | 11/45 | 0.673 |
| Etiology of HCC (HCV/HBV/others 2) | 24/5/16 | 19/4/8 | 38/9/21 | 30/11/15 | 0.743 |
| Child-Pugh classification (Class A/B) | 43/2 | 24/7 | 58/10 | 47/9 | 0.136 |
| Lesion characteristics | |||||
| No. of lesions | 45 | 31 | 68 | 56 | / |
| Location (Left/Right hepatic lobe) | 15/30 | 13/18 | 28/40 | 13/43 | 0.152 |
| Size(s) (diameter(s)) (median (interquartile range), mm) | 14.00 (12.00–16.00) | 17.00 (15.00–20.00) | 17.50 (15.00–23.50) | 20.00 (12.00–26.50) | <0.001 |
| Indicators and Patterns | Characteristics | Group Comparison | p-Value |
|---|---|---|---|
| Size(s) (diameter(s)) of the lesions 2 | / | Early and well differentiated | <0.001 |
| Early and moderately differentiated | <0.001 | ||
| Early and poorly differentiated | <0.001 | ||
| Echogenicity on the grayscale US images | hyper/iso/hypo | total | 0.286 |
| Halo sign | positive/negative | Early (2/43) and well differentiated (13/18) | <0.001 |
| Early and moderately differentiated (46/22) | <0.001 | ||
| Early and poorly differentiated (32/24) | <0.001 | ||
| Well differentiated and moderately differentiated | 0.016 | ||
| Mosaic sign | positive/negative | Early (1/44) and moderately differentiated (24/44) | <0.001 |
| Early and poorly differentiated (20/36) | <0.001 | ||
| Vascularity of AP in Sonazoid CEUS | hyper/iso/hypo | Early (13/6/26) and well differentiated (25/1/5) | <0.001 |
| Early and moderate (56/4/8) | <0.001 | ||
| Early and poorly (50/5/1) | <0.001 | ||
| Vascularity in PP of Sonazoid CEUS | iso/hypo | Early (41/3) and moderate (49/17) | 0.012 |
| Poorly (14/41) and early | <0.001 | ||
| Poorly and well (24/7) | <0.001 | ||
| Poorly and moderate | <0.001 | ||
| Echo in PVP of Sonazoid CEUS | iso/hypo | Early (41/4) and well (12/19) | <0.001 |
| Early and moderate (1/67) | <0.001 | ||
| Early and poorly (0/56) | <0.001 | ||
| Poorly and well | <0.001 | ||
| Moderate and well | <0.001 | ||
| Intense in HBP of Gd-EOB-DTPA MRI | low/iso or high | Early (40/0) and poorly (39/6) | 0.027 |
| Histological Differentiation Grade | Image Indicators and Patterns | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC (95% CI) | |
|---|---|---|---|---|---|---|
| Early | Halo sign (Absence) | 95.6 | 58.7 | 67.0 | 0.769 (0.700–0.837) | |
| Mosaic sign (Absence) | 97.8 | 31.6 | 46.5 | 0.647 (0.567–0.727) | ||
| Hypo in AP | 57.8 | 91.0 | 83.5 | 0.744 (0.651–0.837) | ||
| Iso in PP | 89.1 | 43.5 | 54.0 | 0.666 (0.584–0.749) | ||
| Iso in PVP | 91.1 | 91.6 | 91.5 | 0.911 (0.856–0.967) | ||
| Fully satisfying ”Iso in PP and PVP” | 86.7 | 91.6 | 90.5 | 0.891 (0.828–0.954) | ||
| Fully satisfying ”Iso in PVP and absence of halo sign” | total | 86.7 | 94.8 | 93.0 | 0.908 (0.846–0.969) | |
| Size < 18 mm | 87.2 | 91.8 | 90.0 | 0.900 (0.830–0.971) | ||
| Size =>18 mm | 83.3 | 96.1 | 95.2 | 0.897 (0.720–1.075) | ||
| Poorly differentiated | Halo sign (Presence) | 56.6 | 57.1 | 57.0 | 0.569 (0.479–0.659) | |
| Hyper in AP | 88.7 | 34.0 | 48.5 | 0.609 (0.524–0.690) | ||
| Hypo in PP | 71.7 | 80.3 | 78.0 | 0.750 (0.667–0.833) | ||
| Hypo in PVP | 100.0 | 36.7 | 53.3 | 0.683 (0.608–0.757) | ||
| Hypo in both PP and PVP | 69.8 | 81.6 | 78.5 | 0.747 (0.663–0.831) | ||
| Moderately differentiated 2 | Fully satisfying “Iso in PP and Hypo in PVP” | 70.6 | 77.3 | 75.0 | 0.739 (0.664–0.815) | |
| Well-differentiated 2 | Fully satisfying “Hypo in AP and iso in PP” | 71.0 | 60.4 | 62.0 | 0.657 (0.554–0.759) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Numata, K.; Nakano, M.; Tanabe, M.; Chuma, M.; Nihonmatsu, H.; Nozaki, A.; Ogushi, K.; Luo, W.; Ruan, L.; et al. Diagnostic Value of Imaging Methods in the Histological Four Grading of Hepatocellular Carcinoma. Diagnostics 2020, 10, 321. https://doi.org/10.3390/diagnostics10050321
Wang F, Numata K, Nakano M, Tanabe M, Chuma M, Nihonmatsu H, Nozaki A, Ogushi K, Luo W, Ruan L, et al. Diagnostic Value of Imaging Methods in the Histological Four Grading of Hepatocellular Carcinoma. Diagnostics. 2020; 10(5):321. https://doi.org/10.3390/diagnostics10050321
Chicago/Turabian StyleWang, Feiqian, Kazushi Numata, Masayuki Nakano, Mikiko Tanabe, Makoto Chuma, Hiromi Nihonmatsu, Akito Nozaki, Katsuaki Ogushi, Wen Luo, Litao Ruan, and et al. 2020. "Diagnostic Value of Imaging Methods in the Histological Four Grading of Hepatocellular Carcinoma" Diagnostics 10, no. 5: 321. https://doi.org/10.3390/diagnostics10050321
APA StyleWang, F., Numata, K., Nakano, M., Tanabe, M., Chuma, M., Nihonmatsu, H., Nozaki, A., Ogushi, K., Luo, W., Ruan, L., Okada, M., Otani, M., Inayama, Y., & Maeda, S. (2020). Diagnostic Value of Imaging Methods in the Histological Four Grading of Hepatocellular Carcinoma. Diagnostics, 10(5), 321. https://doi.org/10.3390/diagnostics10050321






