Vitamin D-Binding Protein (Gc-Globulin) in Acute Liver Failure in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrolment
2.2. Study Procedures
2.3. Measurement of Serum Markers
2.4. Data Analysis and Statistics
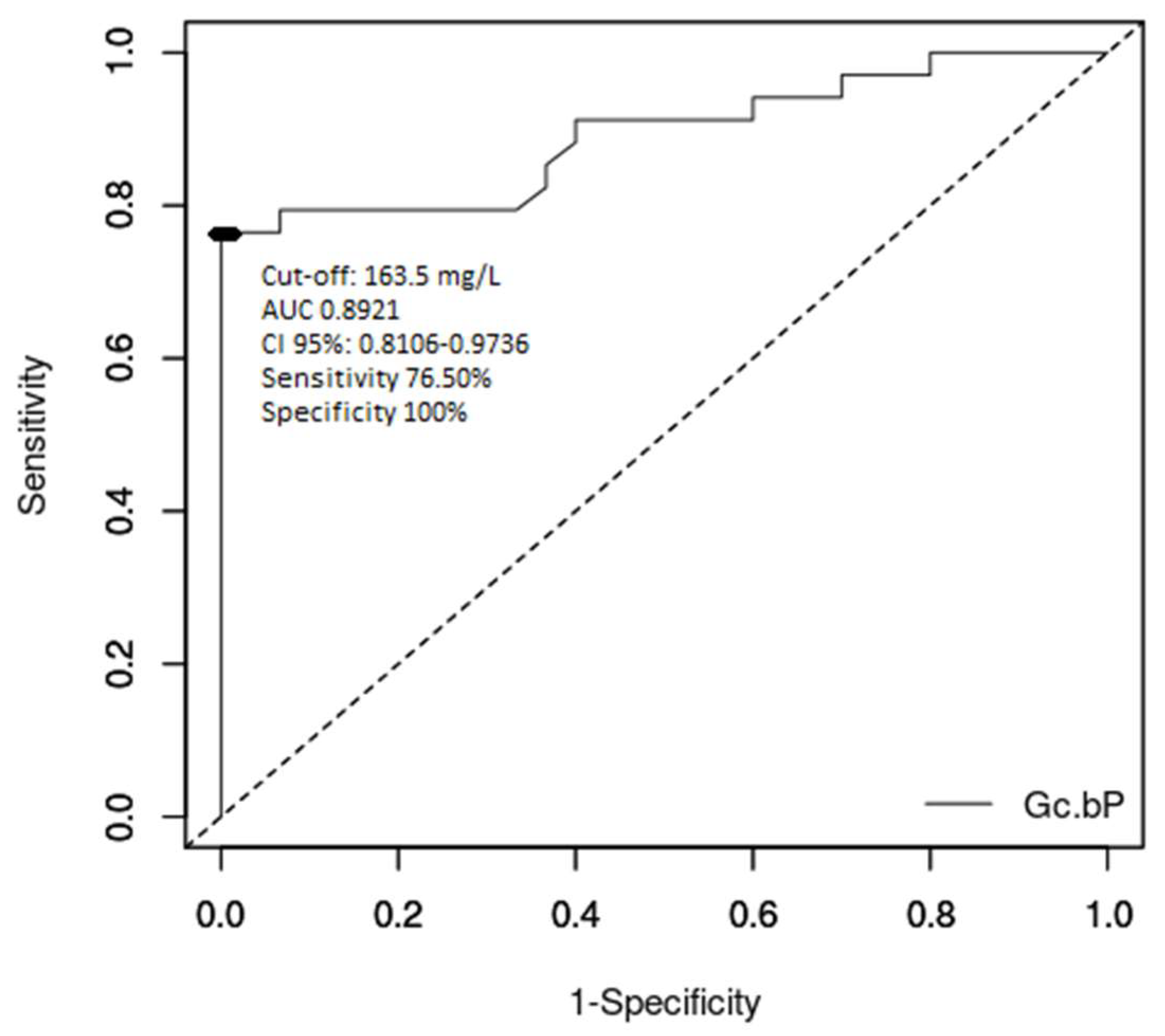
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whitington, P.F.; Alonso, W.A. Fulminant hepatitis and acute liver failure. In Diseases of the Liver and Biliary System in Children, 3rd ed.; Deirdre, K., Ed.; Wiley-Blackwell: Oxford, UK, 2008; pp. 92–123. [Google Scholar]
- Devictor, D.; Tissieres, P.; Afanetti, M.; Debray, D. Acute liver failure in children. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Altinbas, A.; Bechmann, L.P.; Akkiz, H.; Gerken, G.; Canbay, A. Acute liver failure. In Hepatology—A Clinical Textbook, 6th ed.; Mauss, S., Berg, T., Sarazzin, J., Wedemeyer, H., Eds.; Flying Publisher: Germany, 2017; pp. 631–641. [Google Scholar]
- Gazzard, G.B.; Henderson, M.J.; Williams, R. Factor VII levels as a guide to prognosis in fulminant hepatic failure. Gut 1976, 17, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Izumi, S.; Langley, P.G.; Wendon, J.; Ellis, A.J.; Pernambuco, R.; Hughes, R.D.; Williams, R. Coagulation factor V levels as a prognostic indicator in fulminant hepatic failure. Hepatology 1996, 23, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, M.; Ramonet, M.; Cuarterolo, M.; Lopez, S.; Cernadas, C.; Alvarez, F. Prognostic factors in paediatric acute liver failure. Arch. Dis. Child. 2008, 93, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Munoz, S.J.; Stravitz, R.T.; Gabriel, D.A. Coagulopathy of acute liver failure. Clin. Liver Dis. 2009, 13, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Dymock, I.W.; Tucker, J.S.; Woolf, I.L.; Poller, L.; Thomson, J.M. Coagulation studies as a prognostic index in acute liver failure. Br. J. Haematol. 1975, 29, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Yantorno, E.S.; Kremers, K.W.; Ruf, E.A.; Trentadue, J.J.; Podestá, L.G.; Villamil, F.G. MELD is superior to King’s College and Clichy’s criteria to assess prognosis in fulminant hepatic failure. Liver Transpl. 2007, 13, 822–828. [Google Scholar] [CrossRef]
- Özçay, F.; Karadağ-Öncel, E.; Barış, Z.; Canan, O.; Moray, G.; Haberal, M. Etiologies, outcomes, and prognostic factors of pediatric acute liver failure: A single center’s experience in Turkey. Turk. J. Gastroenterol. 2016, 27, 450–457. [Google Scholar] [CrossRef]
- Squires, R.H., Jr. Acute Liver Failure in Children. Semin. Liver Dis. 2008, 28, 153–166. [Google Scholar] [CrossRef]
- Gilbert, P.J.; Moreno, B.J.; Rodríguez, S.M. Aetiology, outcomes and prognostic indicators of paediatric acute liver failure. An. Pediatr. 2018, 88, 61–112. [Google Scholar] [CrossRef]
- O’Grady, J.; Alexander, G.; Hayllar, K.; Williams, R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989, 97, 439–445. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- McPhail, M.J.; Farne, H.; Senvar, N.; Wendon, J.A.; Bernal, W. Ability of King’s College Criteria and Model for End-Stage Liver Disease Scores to Predict Mortality of Patients with Acute Liver Failure: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Schiødt, F.V.; Rossaro, L.; Stravitz, R.T.; Shakil, A.O.; Chung, R.T.; Lee, W.M.; Acute Liver Failure Study Group. Gc-globulin and prognosis in acute liver failure. Liver Transpl. 2005, 11, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Emerson, D.L.; Werner, P.A.M.; Arnaud, P.; Goldschmidt-Clermont, P.; Galbraith, R.M. Decreased serum group-specific component protein levels and complexes with actin in fulminant hepatic necrosis. Hepatology 1985, 5, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt-Clermont, P.; Lee, W.M.; Galbraith, R.M. Proportion of circulating Gc (vitamin D-binding protein) in complexed form: Relation to clinical outcome in fulminant hepatic necrosis. Gastroenterology 1988, 94, 1454–1458. [Google Scholar] [CrossRef]
- Goldschmidt-Clermont, P.J.; Galbraith, R.M.; Emerson, D.L.; Werner, P.A.; Nel, A.E.; Lee, W.M. Accurate quantitation of native Gc protein in serum and estimation of endogenous Gc: G-actin complexes by rocket immunoelectrophoresis. Clin. Chim. Acta 1985, 148, 173–183. [Google Scholar] [CrossRef]
- Favaloro, J.E.; Funk, M.D.; Lippi, G. Pre-analytical Variables in Coagulation Testing Associated with Diagnostic Errors in Haemostasis. Lab. Med. 2012, 43, 1–10. [Google Scholar] [CrossRef]
- Goksuluk, D.; Korkmaz, S.; Zararsiz, G.; Karaagaoglu, E. easyROC: An Interactive Web-tool for ROC Curve Analysis Using R Language Environment. R J. 2016, 8, 213–230. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Nunez-Ramos, R.; Montoro, S.; Bellusci, M.; Del Fresno-Valencia, M.R.; Germán-Díaz, M.; Urruzuno, P.; Medina, E.; Manzanares, J. Acute Liver Failure: Outcome and Value of Pediatric End-Stage Liver Disease Score in Pediatric Cases. Pediatr. Emerg. Care 2018, 34, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C.; D’Agostino, D.E. Pediatric end-stage liver disease score in acute liver failure to assess poor prognosis. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Pop, H.F.; Sarbu, C.; Stefanescu, A.; Bizo, A.; Pop, T.L. Prognostic factors in liver failure in children by discriminant analysis of clinical data. A chemometric approach. Studia UBB Chemia 2015, 60, 101–108. [Google Scholar]
- Pop, T.L.; Grama, A.; Stefanescu, A.; Delean, D.; Aldea, C.; Bizo, A. PELD score as a prognostic factor in fulminant liver failure caused by mushroom poisoning in children. J. Hepatol. 2016, 64 (Suppl. 2), S304–S305. [Google Scholar] [CrossRef]
- Stefanescu, A.; Pop, T.; Stefanescu, H.; Feier, D.; Bizo, A.; Miu, N. Serum creatinine and the presence of encephalopathy at presentation may predict mortality in children with acute liver failure. J. Hepatol. 2013, 58 (Suppl. 1), s417. [Google Scholar] [CrossRef]
- Bernal, W.; Wendon, J. Acute liver failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef]
- Sokol, R.J.; Narkewicz, M.R. Liver and pancreas. In Current Diagnosis & Treatment Pediatrics, 20th ed.; Hay, W., Myron, L., Sondheimer, J.M., Deterding, R.R., Eds.; Mc Graw Hill Education: New York, NY, USA, 2011; pp. 631–651. [Google Scholar]
- Grama, A.; Aldea, C.; Burac, L.; Delean, D.; Boghitoiu, D.; Bulata, B.; Nitescu, V.; Ulmeanu, C.; Pop, T.L. Acute liver failure secondary to toxic exposure in children. Arch. Med. Sci. 2019. [Google Scholar] [CrossRef]
- Grama, A.; Pop, T.L. Treatment of acute liver failure in children. Pediatru.ro 2019, 53, 30–35. [Google Scholar] [CrossRef]
- Grama, A.; Blaga, L.; Nicolescu, A.; Deleanu, C.; Militaru, M.; Cainap, S.S.; Pop, I.; Tita, G.; Sirbe, C.; Fufezan, O.; et al. Novel Mutation in GALT Gene in Galactosemia Patient with Group B Streptococcus Meningitis and Acute Liver Failure. Medicina 2019, 55, 91. [Google Scholar] [CrossRef]
- Hirschfeld, J. Immune-electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta Pathol. Microbiol. Scand. 1959, 47, 160–168. [Google Scholar] [CrossRef]
- Schiodt, F.V. Gc-globulin in liver disease. Dan. Med. Bull. 2008, 55, 131–146. [Google Scholar] [PubMed]
- Dahl, B.; Schiødt, F.V.; Rudolph, S.; Ott, P.; Kiaer, T.; Heslet, L. Trauma stimulates the synthesis of Gc-globulin. Intensive Care Med. 2001, 27, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Wians, F.H., Jr.; Lin, W.; Brown, L.P.; Schiødt, F.V.; Lee, W.M. Immunonephelometric quantification of group-specific component protein in patients with acute liver failure. Liver Transpl. Surg. 1997, 3, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ostapowicz, G.; Fontana, R.J.; Schiødt, F.V.; Larson, A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002, 137, 947–954. [Google Scholar] [CrossRef]
- Schiødt, F.V.; Bondesen, S.; Tygstrup, N. Serial measurements of serum Gc-globulin in acetaminophen intoxication. Eur. J. Gastroenterol. Hepatol. 1995, 7, 635–640. [Google Scholar]
- Schiødt, F.V.; Bondesen, S.; Petersen, I.; Dalhoff, K.; Ott, P.; Tygstrup, N. Admission levels of serum Gc-globulin: Predictive value in fulminant hepatic failure. Hepatology 1996, 23, 713–718. [Google Scholar] [CrossRef]
- Schiødt, F.V.; Ott, P.; Tygstrup, N.; Dahl, B.; Bondesen, S. Temporal profile of total, bound, and free Gc-globulin after acetaminophen overdose. Liver Transpl. 2001, 7, 732–738. [Google Scholar] [CrossRef]
- Antoniades, G.C.; Berry, A.P.; Bruce, M.; Cross, T.J.; Portal, A.J.; Hussain, M.J.; Bernal, W.; Wendon, J.A.; Vergani, D. Actin-Free Gc Globulin: A Rapidly Assessed Biomarker of Organ Dysfunction in Acute Liver Failure and Cirrhosis. Liver Transpl. 2007, 13, 1254–1261. [Google Scholar] [CrossRef]

| Variable | ALF (n = 34) | Controls (n = 30) | p Value |
|---|---|---|---|
| Age (years) | 4.31 ± 4.89 | 6.11 ± 4.26 | 0.124231 |
| Sex: Males | 18 (52.94%) | 18 (60%) | 0.5700 |
| Aetiology of ALF: | - | ||
| Metabolic disorders | 9 (26.47%) | - | |
| Autoimmune hepatitis | 8 (23.53%) | - | |
| Toxic | 7 (20.59%) | - | |
| Infections | 6 (17.65%) | - | |
| Unknown | 4 (11.76%) | - | |
| AST (UI/dL) | 1466.53 ± 2203.44 | 32.77 ± 8.02 | 0.000717 |
| ALT (UI/dL) | 1265.24 ± 1517.38 | 20.03 ± 8.17 | 0.000032 |
| Factor V (%) | 37.25 ± 26.75 | 86.62 ± 16.11 | <0.000001 |
| Factor VII (%) | 25.74 ± 24.52 | 87.56 ± 21.51 | <0.000001 |
| INR | 4.63 ± 3.17 | 1.29 ± 0.21 | <0.000001 |
| % Prothrombin | 29.23 ± 20.81 | 83.84 ± 19.05 | <0.000001 |
| Gc-globulin (mg/L) | 151.57 ± 171.87 | 498.63 ± 252.50 | <0.000001 |
| PELD score | 22.57 ± 13.94 | - | - |
| Evolution: | - | ||
| Alive | 20 (58.82%) | 30 | |
| Liver transplantation | 1 (2.94%) | - | |
| Deceased | 13 (38.24%) | - |
| Parameters | Deceased or Liver Transplanted (n = 14) | Alive (n = 20) | p Value |
|---|---|---|---|
| AST (UI/dL) | 1406.71 ± 1749.22 | 1508.40 ± 2516.80 | 0.897028 |
| ALT (UI/dL) | 1198.43 ± 1680.04 | 1312.00 ± 1436.04 | 0.833716 |
| Total Bilirubin (mg/dL) | 8.83 ± 7.71 | 7.07 ± 7.22 | 0.503015 |
| Direct Bilirubin (mg/dL) | 5.50 ± 4.26 | 5.36 ± 5.88 | 0.937538 |
| Factor V (%) | 18.93 ± 19.96 | 43.90 ± 26.98 | 0.008193 |
| Factor VII (%) | 10.72 ± 10.22 | 38.89 ± 26.02 | 0.000724 |
| INR | 7.06 ± 3.20 | 2.93 ± 1.75 | 0.000031 |
| % Prothrombin | 14.86 ± 14.06 | 39.29 ± 18.92 | 0.000267 |
| Gc-globulin (mg/L) | 59.34 ± 33.73 | 216.12 ± 199.69 | 0.006763 |
| PELD score | 30.06 ± 15.55 | 16.76 ± 9.35 | 0.005336 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grama, A.; Burac, L.; Aldea, C.O.; Bulata, B.; Delean, D.; Samasca, G.; Abrudan, C.; Sirbe, C.; Pop, T.L. Vitamin D-Binding Protein (Gc-Globulin) in Acute Liver Failure in Children. Diagnostics 2020, 10, 278. https://doi.org/10.3390/diagnostics10050278
Grama A, Burac L, Aldea CO, Bulata B, Delean D, Samasca G, Abrudan C, Sirbe C, Pop TL. Vitamin D-Binding Protein (Gc-Globulin) in Acute Liver Failure in Children. Diagnostics. 2020; 10(5):278. https://doi.org/10.3390/diagnostics10050278
Chicago/Turabian StyleGrama, Alina, Lucia Burac, Cornel Olimpiu Aldea, Bogdan Bulata, Dan Delean, Gabriel Samasca, Carmen Abrudan, Claudia Sirbe, and Tudor Lucian Pop. 2020. "Vitamin D-Binding Protein (Gc-Globulin) in Acute Liver Failure in Children" Diagnostics 10, no. 5: 278. https://doi.org/10.3390/diagnostics10050278
APA StyleGrama, A., Burac, L., Aldea, C. O., Bulata, B., Delean, D., Samasca, G., Abrudan, C., Sirbe, C., & Pop, T. L. (2020). Vitamin D-Binding Protein (Gc-Globulin) in Acute Liver Failure in Children. Diagnostics, 10(5), 278. https://doi.org/10.3390/diagnostics10050278







