Role of Peroral Cholangioscopy in the Diagnosis of Primary Sclerosing Cholangitis
Abstract
1. Introduction
2. Radiological Images of PSC
3. Excluding Other Causes of Biliary Stricture
4. POCS for Diagnosing PSC
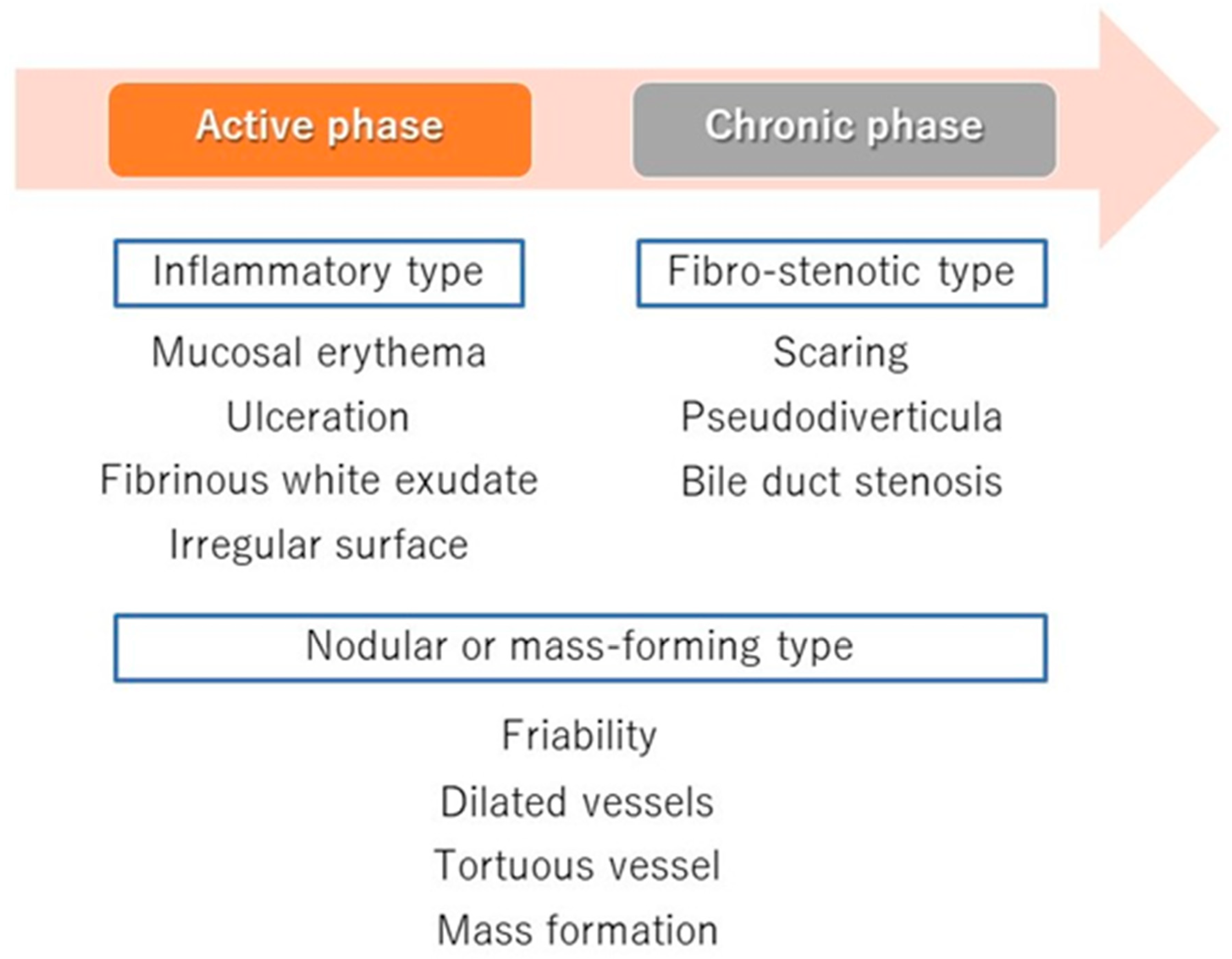
5. The Phases of PSC and Cholangioscopic Findings in Each Phase
6. Diagnosis and Classification of PSC Using POCS
Funding
Conflicts of Interest
References
- Fricker, Z.P.; Lichtenstein, D.R. Primary Sclerosing Cholangitis: A Concise Review of Diagnosis and Management. Dig. Dis. Sci. 2019, 64, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary sclerosing cholangitis—A comprehensive review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.N.; LaRusso, N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Notohara, K.; Tazuma, S.; Tanaka, A.; Isayama, H.; Tsuyuguchi, T.; Mori, T.; Takikawa, H. The 2016 diagnostic criteria for primary sclerosing cholangitis. J. Gastroenterol. 2017, 52, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Barkin, J.A.; Levy, C.; Souto, E.O. Endoscopic Management of Primary Sclerosing Cholangitis. Ann. Hepatol. 2017, 16, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Elmunzer, B.J.; Dwamena, B.A.; Higgins, P.D.R. Primary Sclerosing Cholangitis: Meta-Analysis of Diagnostic Performance of MR Cholangiopancreatography. Radiology 2010, 256, 387–396. [Google Scholar] [CrossRef]
- Patil, K.; Ricciuto, A.; Alsharief, A.; Al-Rayahi, J.; Amirabadi, A.; Church, P.C.; Kamath, B.M.; Greer, M.C. Magnetic Resonance Cholangiopancreatography Severity Predicts Disease Outcomes in Pediatric Primary Sclerosing Cholangitis: A Reliability and Validity Study. Hepatol. Commun. 2019, 4, 208–218. [Google Scholar] [CrossRef]
- Belle, A.; Laurent, V.; Pouillon, L.; Baumann, C.; Orry, X.; Lopez, A.; Rousseau, H.; Bronowicki, J.P.; Peyrin-Biroulet, L. Systematic screening for primary sclerosing cholangitis with magnetic resonance cholangiography in inflammatory bowel disease. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2018, 50, 1012–1018. [Google Scholar] [CrossRef]
- Tenca, A.; Mustonen, H.; Lind, K.; Lantto, E.; Kolho, K.L.; Boyd, S.; Arola, J.; Jokelainen, K.; Farkkila, M. The role of magnetic resonance imaging and endoscopic retrograde cholangiography in the evaluation of disease activity and severity in primary sclerosing cholangitis. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 2329–2339. [Google Scholar] [CrossRef]
- Talwalkar, J.A.; Angulo, P.; Johnson, C.D.; Petersen, B.T.; Lindor, K.D. Cost-minimization analysis of MRC versus ERCP for the diagnosis of primary sclerosing cholangitis. Hepatology 2004, 40, 39–45. [Google Scholar] [CrossRef]
- Meagher, S.; Yusoff, I.; Kennedy, W.; Martel, M.; Adam, V.; Barkun, A. The roles of magnetic resonance and endoscopic retrograde cholangiopancreatography (MRCP and ERCP) in the diagnosis of patients with suspected sclerosing cholangitis: A cost-effectiveness analysis. Endoscopy 2007, 39, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Fung, B.M.; Tabibian, J.H. Biliary endoscopy in the management of primary sclerosing cholangitis and its complications. Liver Res. 2019, 3, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Ohara, H.; Sano, H.; Aoki, S.; Kobayashi, S.; Okamoto, T.; Imai, H.; Nomura, T.; Joh, T.; Itoh, M. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest. Endosc. 2004, 60, 937–944. [Google Scholar] [CrossRef]
- Kamisawa, T.; Nakazawa, T.; Tazuma, S.; Zen, Y.; Tanaka, A.; Ohara, H.; Muraki, T.; Inui, K.; Inoue, D.; Nishino, T.; et al. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J. Hepato-Biliary-Pancreat. Sci. 2019, 26, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, K.S.; Das, P.; Gunjan, D.; Srivastava, D.N.; Garg, P.K. IgG4-Related Sclerosing Cholangitis: A Clinical and Imaging Review. AJR Am. J. Roentgenol. 2019, 213, 1221–1231. [Google Scholar] [CrossRef]
- Tanaka, A. IgG4-Related Sclerosing Cholangitis and Primary Sclerosing Cholangitis. Gut Liver 2019, 13, 300–307. [Google Scholar] [CrossRef]
- Ponsioen, C.Y.; Arnelo, U.; Bergquist, A.; Rauws, E.A.; Paulsen, V.; Cantu, P.; Parzanese, I.; De Vries, E.M.; van Munster, K.N.; Said, K.; et al. No Superiority of Stents vs Balloon Dilatation for Dominant Strictures in Patients With Primary Sclerosing Cholangitis. Gastroenterology 2018, 155, 752–759. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Bowlus, C.L.; Yang, G.; Leung, P.S.C.; Gershwin, M.E. Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis (PSC): A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 134–149. [Google Scholar] [CrossRef]
- Chapman, R.W.; Williamson, K.D. Are Dominant Strictures in Primary Sclerosing Cholangitis a Risk Factor for Cholangiocarcinoma? Curr. Hepatol. Rep. 2017, 16, 124–129. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Strassburg, C.P.; Trivedi, P.J.; Hirschfield, G.M.; Trivedi, P.J.; Bergquist, A.; Said, K.; Imam, M.; Lazaridis, K.N.; Juran, B.D.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, A.; Ekbom, A.; Olsson, R.; Kornfeldt, D.; Lööf, L.; Danielsson, Å.; Hultcrantz, R.; Lindgren, S.; Prytz, H.; Sandberg-Gertzén, H.; et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J. Hepatol. 2002, 36, 321–327. [Google Scholar] [CrossRef]
- Isayama, H.; Tazuma, S.; Kokudo, N.; Tanaka, A.; Tsuyuguchi, T.; Nakazawa, T.; Notohara, K.; Mizuno, S.; Akamatsu, N.; Serikawa, M.; et al. Clinical guidelines for primary sclerosing cholangitis 2017. J. Gastroenterol. 2018, 53, 1006–1034. [Google Scholar] [CrossRef]
- Nakazawa, T.; Naitoh, I.; Hayashi, K.; Miyabe, K.; Simizu, S.; Joh, T. Diagnosis of IgG4-related sclerosing cholangitis. World J. Gastroenterol. 2013, 19, 7661–7670. [Google Scholar] [CrossRef]
- Aabakken, L.; Karlsen, T.H.; Albert, J.; Arvanitakis, M.; Chazouilleres, O.; Dumonceau, J.M.; Farkkila, M.; Fickert, P.; Hirschfield, G.M.; Laghi, A.; et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy 2017, 49, 588–608. [Google Scholar] [CrossRef]
- Nakazawa, T.; Naitoh, I.; Hayashi, K.; Okumura, F.; Miyabe, K.; Yoshida, M.; Yamashita, H.; Ohara, H.; Joh, T. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. J. Gastroenterol. 2012, 47, 79–87. [Google Scholar] [CrossRef]
- Campbell, W.L.; Peterson, M.S.; Federle, M.P.; Siqueira, E.S.; Slivka, A.; Grazioli, L.; Ichikawa, T.; Oliver, J.H., 3rd; Kim, T.; Li, W. Using CT and cholangiography to diagnose biliary tract carcinoma complicating primary sclerosing cholangitis. AJR Am. J. Roentgenol. 2001, 177, 1095–1100. [Google Scholar] [CrossRef]
- Njei, B.; McCarty, T.R.; Varadarajulu, S.; Navaneethan, U. Cost utility of ERCP-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastrointest. Endosc. 2017, 85, 773–781.e10. [Google Scholar] [CrossRef]
- Tischendorf, J.J.; Kruger, M.; Trautwein, C.; Duckstein, N.; Schneider, A.; Manns, M.P.; Meier, P.N. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy 2006, 38, 665–669. [Google Scholar] [CrossRef]
- Arnelo, U.; von Seth, E.; Bergquist, A. Prospective evaluation of the clinical utility of single-operator peroral cholangioscopy in patients with primary sclerosing cholangitis. Endoscopy 2015, 47, 696–702. [Google Scholar] [CrossRef]
- Kalaitzakis, E.; Sturgess, R.; Kaltsidis, H.; Oppong, K.; Lekharaju, V.; Bergenzaun, P.; Vlavianos, P.; Sharma, H.; Westaby, D.; Webster, G.J. Diagnostic utility of single-user peroral cholangioscopy in sclerosing cholangitis. Scand. J. Gastroenterol. 2014, 49, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Kamisawa, T.; Igarashi, Y.; Kawakami, H.; Yasuda, I.; Itokawa, F.; Kishimoto, Y.; Kuwatani, M.; Doi, S.; Hara, S.; et al. The role of peroral video cholangioscopy in patients with IgG4-related sclerosing cholangitis. J. Gastroenterol. 2013, 48, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Sandha, G.; D’Souza, P.; Halloran, B.; Montano-Loza, A.J. A Cholangioscopy-Based Novel Classification System for the Phenotypic Stratification of Dominant Bile Duct Strictures in Primary Sclerosing Cholangitis-the Edmonton Classification. J. Can. Assoc. Gastroenterol. 2018, 1, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Hasan, M.K.; Lourdusamy, V.; Njei, B.; Varadarajulu, S.; Hawes, R.H. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: A systematic review. Gastrointest. Endosc. 2015, 82, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cuadrado-Robles, E.; Deprez, P.H. Indications for Single-Operator Cholangioscopy and Pancreatoscopy: An Expert Review. Curr. Treat. Options Gastroenterol. 2019, 17, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Eccles, J.; Thiesen, A.; Sandha, G. Single-operator cholangioscopy for diagnosis of cholangioadenoma (bile duct adenoma) and its potential impact on surgical management. Endosc. Int. Open 2018, 6, 1312–1316. [Google Scholar] [CrossRef]
- Zimmer, V.; Lammert, F. Positioning cholangioscopy in bile duct stone management: Mind the technology gap. Frontline Gastroenterol. 2018, 9, 315–316. [Google Scholar] [CrossRef]
- Nakai, Y.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; Kogure, H.; et al. Management of Difficult Bile Duct Stones by Large Balloon, Cholangioscopy, Enteroscopy and Endosonography. Gut Liver 2019. [Google Scholar] [CrossRef]
- Kaura, K.; Sawas, T.; Bazerbachi, F.; Storm, A.C.; Martin, J.A.; Gores, G.J.; Abu Dayyeh, B.K.; Topazian, M.D.; Levy, M.J.; Petersen, B.T.; et al. Cholangioscopy Biopsies Improve Detection of Cholangiocarcinoma When Combined with Cytology and FISH, but Not in Patients with PSC. Dig. Dis. Sci. 2019. [Google Scholar] [CrossRef]
- Parsa, N.; Khashab, M.A. The Role of Peroral Cholangioscopy in Evaluating Indeterminate Biliary Strictures. Clin. Endosc. 2019, 52, 556–564. [Google Scholar] [CrossRef]
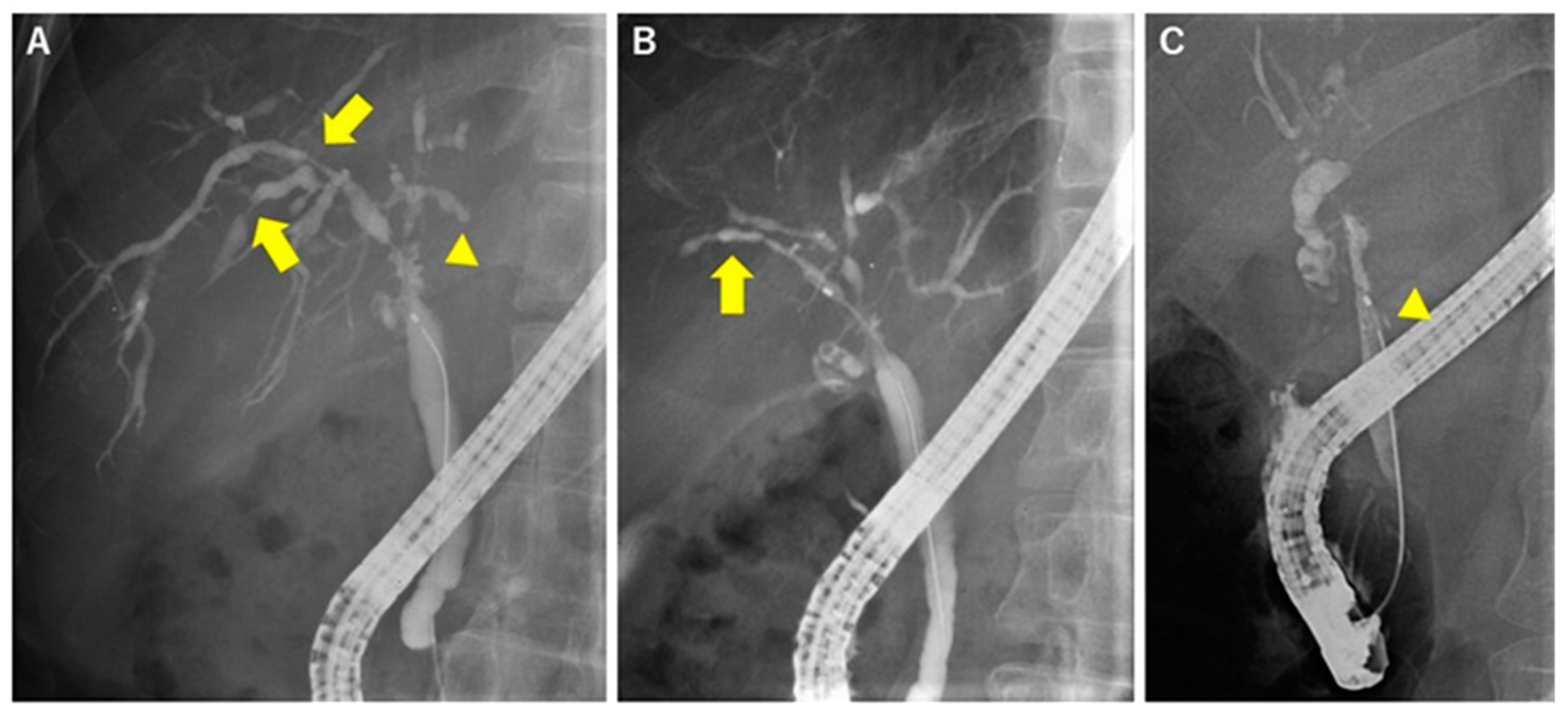
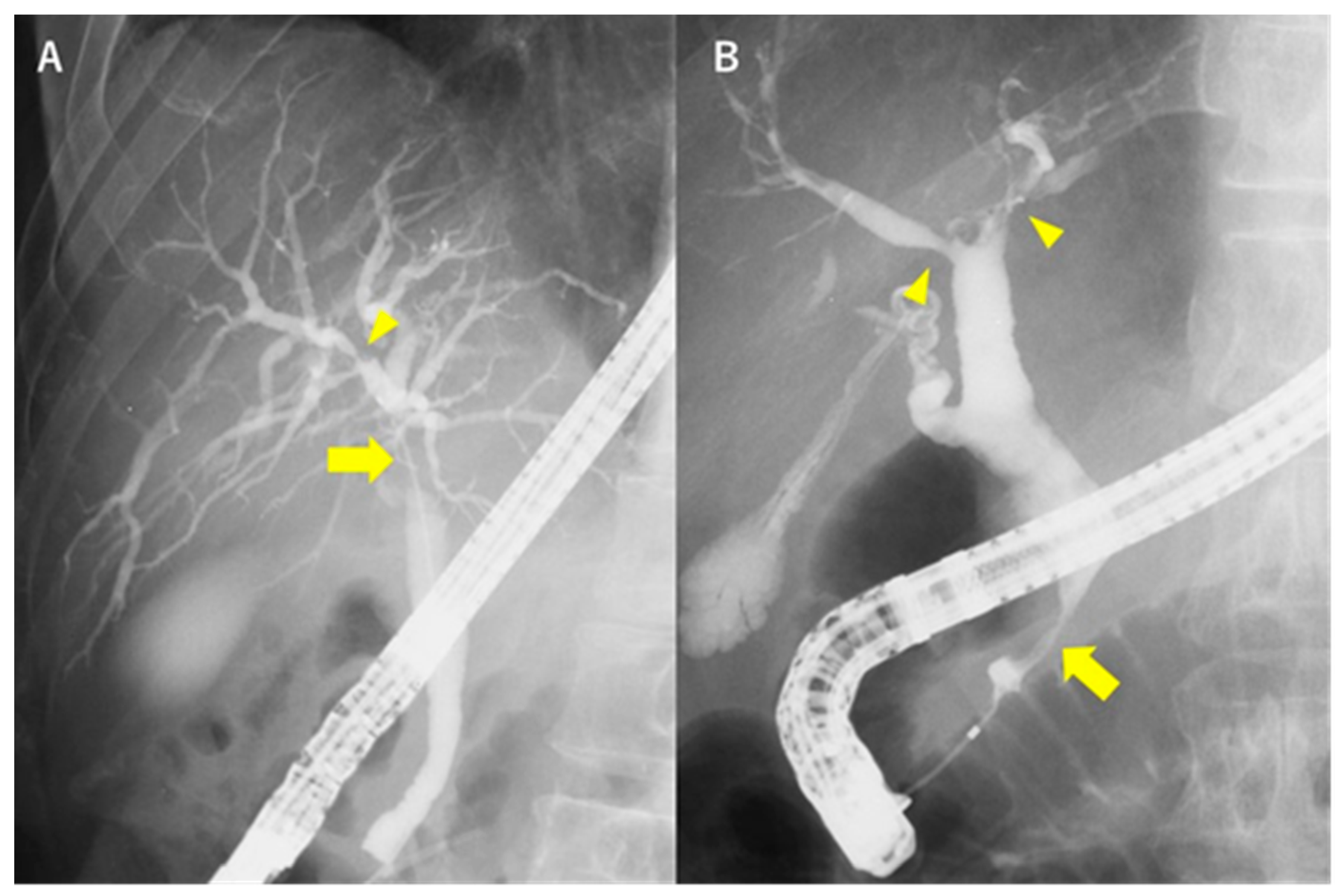
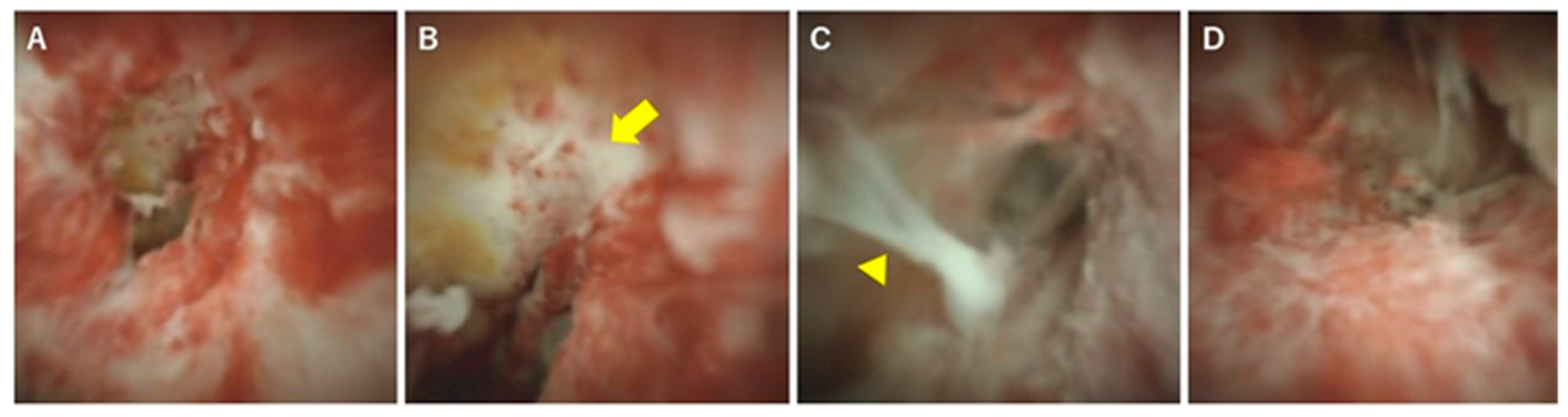

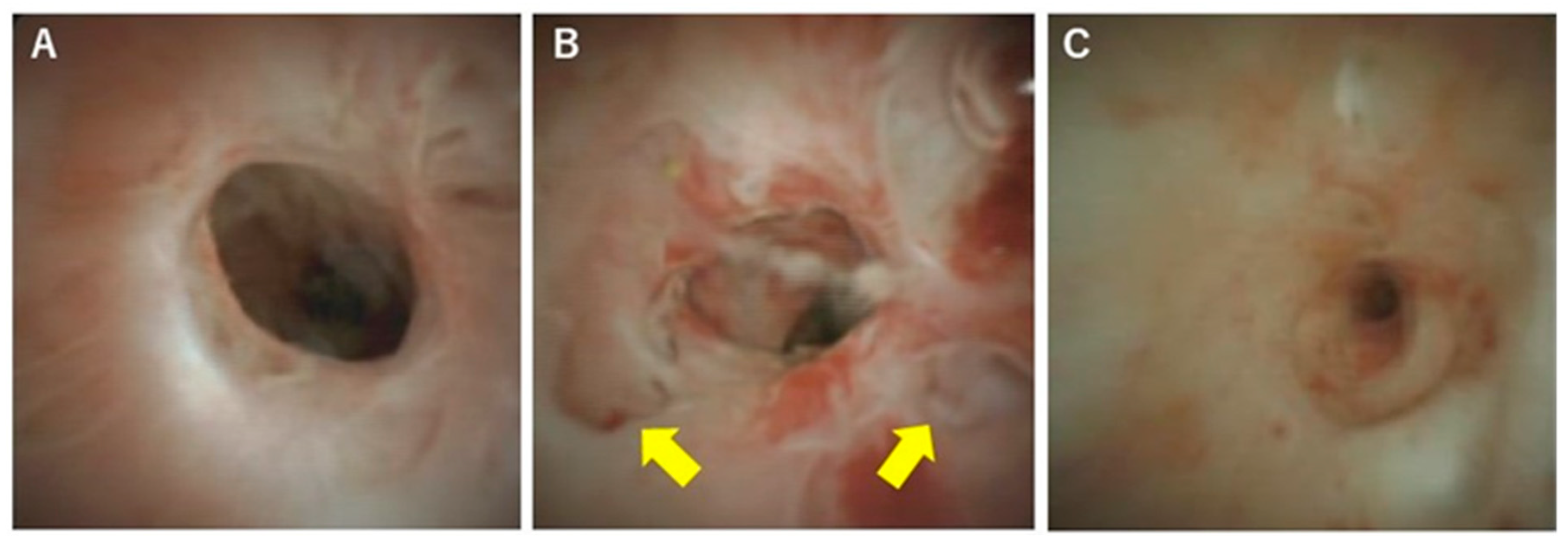
| Causes | Diseases and Pathogens | Diagnosis |
|---|---|---|
| Chronic obstruction | Choledocholithiasis | US, MRC/ERC |
| Idiophathic biliary strictures | Exclusion diagnosis | |
| Neoplasms (benigh and malignancy) | US, CT/MRI, ERC with biopsy | |
| Congenital | Caroli’s disease | History, CT/MRI |
| Cystic fibrosis | Sweat chloride ion, family history, CFTR * gene | |
| Immunologic | Eosinophilic cholangitis | CT/MRC, ERC with biopsy |
| Graft-versus-host disease | History, liver biopsy | |
| Infections | Bacteria (Recurrent pyogenic cholangitis) | History, CT/MRI |
| Virus (cytomeralovirus and HIV *) | Serology, immunological investigations | |
| Parasite | CT/MRI, ERC, serology | |
| Infiltrative disorders | Systemic vasculitis | Biopsy, serum ANCAs *, angiography |
| Amyloidosis | Symptoms, liver biopsy | |
| Sarcoidosis | Serum ACE *, liver biopsy | |
| Systemic mastocytosis | Bone marrow biopsy | |
| Heypereosinophilic syndrome | Eosinophilia, bone marrow biopsy | |
| Hodgkin’s disease | CT/MRI, serum sIL-2R *, histology | |
| Ischemic | Vascular trauma | History, CT/MRI |
| Anastomotic strictures in liver graft | History, CT/MRI | |
| Transcatheter arterial embolization | History | |
| Toxic | Alcohol, formaldehyde, hypertonic saline | History |
| Intraarterial chemotherapy | History | |
| Trauma | Accident, Surgery, and ERCP | History, CT/MRI |
| Radiation injury | History |
| Favors PSC | Characteristic | Favors IgG4-SC |
|---|---|---|
| Short, multiple | Length of stricture | Long |
| Rare | Stricture of intrapancreatic bile duct | Often |
| Pruning | Intrahepatic bile ducts | Dilating |
| Author | Year | Number of Patients | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Tischendorf et al. | 2006 | 53 | 92% | 93% | 93% | 79% | 97% |
| Kalaitzakis et al. | 2014 | 52 (including 4 IgG4-SC) | 50% | 100% | 88% | 100% | 87% |
| Arnelo et al. | 2015 | 47 | 33% | 100% | 96% | 100% | 95% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, T.; Ushio, M.; Takahashi, S.; Yamagata, W.; Takasaki, Y.; Suzuki, A.; Okawa, Y.; Ochiai, K.; Tomishima, K.; Ishii, S.; et al. Role of Peroral Cholangioscopy in the Diagnosis of Primary Sclerosing Cholangitis. Diagnostics 2020, 10, 268. https://doi.org/10.3390/diagnostics10050268
Fujisawa T, Ushio M, Takahashi S, Yamagata W, Takasaki Y, Suzuki A, Okawa Y, Ochiai K, Tomishima K, Ishii S, et al. Role of Peroral Cholangioscopy in the Diagnosis of Primary Sclerosing Cholangitis. Diagnostics. 2020; 10(5):268. https://doi.org/10.3390/diagnostics10050268
Chicago/Turabian StyleFujisawa, Toshio, Mako Ushio, Sho Takahashi, Wataru Yamagata, Yusuke Takasaki, Akinori Suzuki, Yoshihiro Okawa, Kazushige Ochiai, Ko Tomishima, Shigeto Ishii, and et al. 2020. "Role of Peroral Cholangioscopy in the Diagnosis of Primary Sclerosing Cholangitis" Diagnostics 10, no. 5: 268. https://doi.org/10.3390/diagnostics10050268
APA StyleFujisawa, T., Ushio, M., Takahashi, S., Yamagata, W., Takasaki, Y., Suzuki, A., Okawa, Y., Ochiai, K., Tomishima, K., Ishii, S., Saito, H., & Isayama, H. (2020). Role of Peroral Cholangioscopy in the Diagnosis of Primary Sclerosing Cholangitis. Diagnostics, 10(5), 268. https://doi.org/10.3390/diagnostics10050268





