Impact of Gene Polymorphisms in GAS5 on Urothelial Cell Carcinoma Development and Clinical Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Ethics Statement
2.2. Genomic DNA Isolation and the Determination of Genotypes
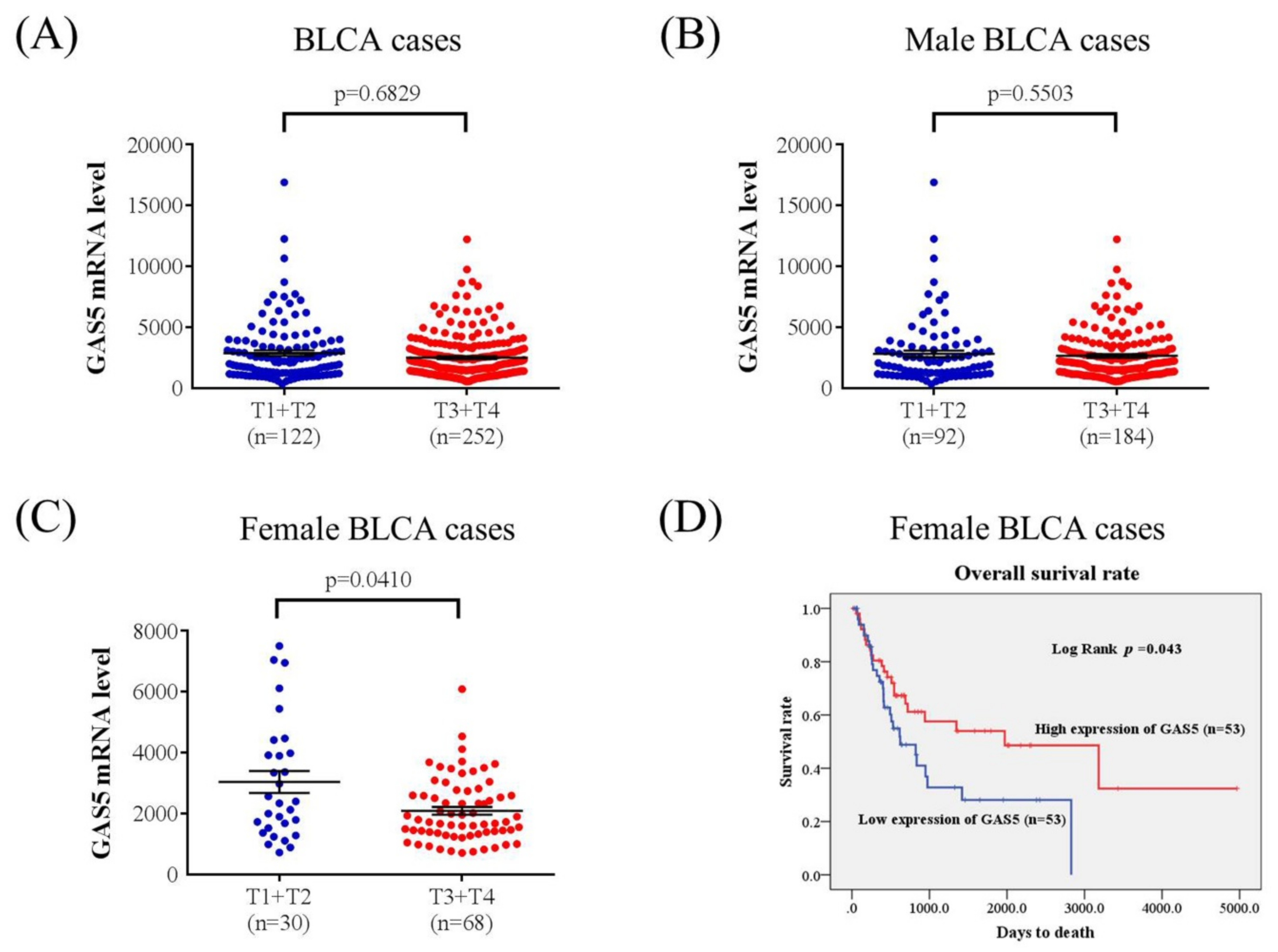
2.3. GAS5 Expression Analysis of the Cancer Genome Atlas
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ajjimaporn, A.; Botsford, T.; Garrett, S.H.; Sens, M.A.; Zhou, X.D.; Dunlevy, J.R.; Sens, D.A.; Somji, S. ZIP8 expression in human proximal tubule cells, human urothelial cells transformed by Cd+2 and As+3 and in specimens of normal human urothelium and urothelial cancer. Cancer Cell Int. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Sahni, S.; Vulisha, A.K.; Gumpeni, R.; Shah, R.; Talwar, A. Pulmonary manifestations of urothelial carcinoma of the bladder. Respir. Med. 2017, 128, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Krege, S.; Lin, C.C.; Hahn, N.; Ecke, T.H.; Moshier, E.; Sonpavde, G.; Godbold, J.; Oh, W.K.; Bamias, A. Cisplatin-based combination chemotherapy in septuagenarians with metastatic urothelial cancer. Urol. Oncol. 2014, 32, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsieh, M.J.; Lin, C.W.; Chuang, C.Y.; Liu, Y.F.; Yeh, C.M.; Yang, S.F. Impact of HOTAIR Gene Polymorphism and Environmental Risk on Oral Cancer. J. Dent. Res. 2018, 97, 717–724. [Google Scholar] [CrossRef]
- Yu, Y.; Hann, S.S. Novel Tumor Suppressor lncRNA Growth Arrest-Specific 5 (GAS5) In Human Cancer. Onco Targets Ther. 2019, 12, 8421–8436. [Google Scholar] [CrossRef]
- Su, S.C.; Reiter, R.J.; Hsiao, H.Y.; Chung, W.H.; Yang, S.F. Functional Interaction between Melatonin Signaling and Noncoding RNAs. Trends Endocrinol. Metab. 2018, 29, 435–445. [Google Scholar] [CrossRef]
- Avgeris, M.; Tsilimantou, A.; Levis, P.K.; Tokas, T.; Sideris, D.C.; Stravodimos, K.; Ardavanis, A.; Scorilas, A. Loss of GAS5 tumour suppressor lncRNA: An independent molecular cancer biomarker for short-term relapse and progression in bladder cancer patients. Br. J. Cancer 2018, 119, 1477–1486. [Google Scholar] [CrossRef]
- Yu, F.; Zheng, J.; Mao, Y.; Dong, P.; Lu, Z.; Li, G.; Fan, X. Long Non-coding RNA Growth Arrest-specific Transcript 5 (GAS5) Inhibits Liver Fibrogenesis through a Mechanism of Competing Endogenous RNA. J. Biol. Chem. 2015, 290, 28286–28298. [Google Scholar] [CrossRef]
- Shen, Z.; She, Q. Association Between the Deletion Allele of Ins/Del Polymorphism (Rs145204276) in the Promoter Region of GAS5 with the Risk of Atherosclerosis. Cell Physiol. Biochem. 2018, 49, 1431–1443. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.Y.; Li, X.; Yuan, X.Q.; Yang, Y.L.; Zhu, K.W.; Zeng, H.; Li, X.-L.; Cao, S.; Zhou, H.-H.; et al. Long non-coding RNA GAS5 polymorphism predicts a poor prognosis of acute myeloid leukemia in Chinese patients via affecting hematopoietic reconstitution. Leuk. Lymphoma 2017, 58, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wang, S.S.; Yang, C.K.; Li, J.R.; Chen, C.S.; Hung, S.C.; Chiu, K.-H.; Cheng, C.-L.; Ou, Y.-C.; Yang, S.-F. Impact of GAS5 genetic polymorphism on prostate cancer susceptibility and clinicopathologic characteristics. Int. J. Med. Sci. 2019, 16, 1424–1429. [Google Scholar] [CrossRef]
- Rakhshan, A.; Esmaeili, M.H.; Kahaei, M.S.; Taheri, M.; Omrani, M.D.; Noroozi, R.; Ghafouri-Fard, S. A Single Nucleotide Polymorphism in GAS5 lncRNA is Associated with Risk of Bladder Cancer in Iranian Population. Pathol. Oncol. Res. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Chou, Y.E.; Li, J.R.; Chen, C.S.; Lin, C.Y.; Chang, L.W.; Chiu, K.-Y.; Cheng, C.-L.; Ou, Y.-C.; Wang, S.-S.; et al. Functional genetic variant of WW domain containing oxidoreductase gene associated with urothelial cell carcinoma clinicopathologic characteristics and long-term survival. Urol. Oncol. 2020, 38, e41–e49. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Wang, S.S.; Li, J.R.; Chen, C.S.; Lin, C.Y.; Chang, L.W.; Chiu, K.-Y.; Cheng, C.-L.; Ou, Y.-C.; Yang, S.-F. Impact of RAGE polymorphisms on urothelial cell carcinoma clinicopathologic characteristics and long-term survival. Urol. Oncol. 2019, 37, e9–e17. [Google Scholar] [CrossRef]
- Su, S.; Chien, M.; Lin, C.; Chen, M.; Yang, S. RAGE gene polymorphism and environmental factor in the risk of oral cancer. J. Dent. Res. 2015, 94, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.R.; Chou, Y.E.; Liu, Y.F.; Hsueh, K.C.; Lee, H.L.; Yang, S.F.; Su, S.-C. Association of lncRNA H19 Gene Polymorphisms with the Occurrence of Hepatocellular Carcinoma. Genes 2019, 10, e506. [Google Scholar] [CrossRef]
- Tung, M.C.; Wen, Y.C.; Wang, S.S.; Lin, Y.W.; Liu, Y.C.; Yang, S.F.; Chien, M.-H. Dopamine receptor D2 genetic variations is associated with the risk and clinicopathological variables of urothelial cell carcinoma in a Taiwanese population. Int. J. Med. Sci. 2018, 15, 1187–1193. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, X.; Liu, D. Overexpression of long noncoding RNA GAS5 suppresses tumorigenesis and development of gastric cancer by sponging miR-106a-5p through the Akt/mTOR pathway. Biol. Open 2019, 8, 041343. [Google Scholar] [CrossRef]
- Krell, J.; Frampton, A.E.; Mirnezami, R.; Harding, V.; De Giorgio, A.; Alonso, L.R.; Cohen, P.; Ottaviani, S.; Colombo, T.; Jacob, J.; et al. Growth arrest-specific transcript 5 associated snoRNA levels are related to p53 expression and DNA damage in colorectal cancer. PLoS ONE 2014, 9, e98561. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Zhao, Y.; Jin, Y.; An, L.; Wu, B.; Liu, Z.; Chen, X.; Zhou, H.; Wang, H.; et al. Genetic polymorphisms of long non-coding RNA GAS5 predict platinum-based concurrent chemoradiotherapy response in nasopharyngeal carcinoma patients. Oncotarget 2017, 8, 62286–62297. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Su, Z.; Fu, W.; Cui, Z.; Jiang, X.; Tai, S. Altered expression of long non-coding RNA GAS5 in digestive tumors. Biosci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Hu, S.; Wang, S.; Zhou, X.; Zhang, Q.; Wang, C.; Zhao, X.; Zhou, W.; Zhang, S.; Li, C.; et al. Association between indel polymorphism in the promoter region of lncRNA GAS5 and the risk of hepatocellular carcinoma. Carcinogenesis 2015, 36, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Aminian, K.; Mashayekhi, F.; Mirzanejad, L.; Salehi, Z. A functional genetic variant in GAS5 lncRNA (rs145204276) modulates p27(Kip1) expression and confers risk for gastric cancer. Br. J. Biomed. Sci. 2019, 76, 83–85. [Google Scholar] [CrossRef]
- Xu, L.; Xia, C.; Xue, B.; Sheng, F.; Xiong, J.; Wang, S. A promoter variant of lncRNA GAS5 is functionally associated with the development of osteosarcoma. J. Bone Oncol. 2018, 12, 23–26. [Google Scholar] [CrossRef]
| Variable | Controls (n = 860) n (%) | Patients (n = 430) n (%) | p-Value |
|---|---|---|---|
| Age (years) | |||
| Mean ± S.D. | 57.2 ± 10 | 68.6 ± 11.8 | <0.001 |
| Gender | 0.365 | ||
| Female | 296 (34.4%) | 159 (37.0%) | |
| Male | 564 (65.6%) | 271 (63.0%) | |
| Tobacco consumption | 0.132 | ||
| No | 562 (65.3%) | 299 (69.5%) | |
| Yes | 298 (34.7%) | 131 (30.4%) | |
| Stage | |||
| Non muscle invasive tumor (pTa–pT1) | 235 (54.7%) | ||
| Muscle invasive tumor (pT2–pT4) | 195 (45.3%) | ||
| Tumor T status | |||
| Ta | 90 (20.9%) | ||
| T1–T4 | 340 (79.1%) | ||
| Lymph node status | |||
| N0 | 379 (88.1%) | ||
| N1 + N2 | 51 (11.9%) | ||
| Metastasis | |||
| M0 | 416 (96.7%) | ||
| M1 | 14 (3.3%) | ||
| Histopathologic grading | |||
| Low grade | 53 (12.3%) | ||
| High grade | 377 (87.7%) |
| Variable | Controls (n = 860) n (%) | Patients (n = 430) n (%) | OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| rs145204276 | ||||
| Ins/Ins | 355 (41.3%) | 191 (44.4%) | 1.000 | 1.000 |
| Ins/Del | 388 (45.1%) | 191 (44.4%) | 0.915 (0.715–1.171) | 0.948 (0.678–1.324) |
| Del/Del | 117 (13.6%) | 48 (11.2%) | 0.763 (0.522–1.114) | 0.737 (0.435–1.247) |
| Ins/Del + Del/Del | 505 (58.7%) | 239 (55.6%) | 0.880 (0.696–1.111) | 0.900 (0.654–1.238) |
| rs55829688 | ||||
| TT | 412 (47.9%) | 208 (48.4%) | 1.000 (reference) | 1.000 (reference) |
| TC | 354 (41.2%) | 187 (43.5%) | 1.046 (0.820–1.335) | 0.948 (0.682–1.319) |
| CC | 94 (10.9%) | 35 (8.1%) | 0.738 (0.483–1.125) | 0.567 (0.304–1.055) |
| TC + CC | 448 (52.1%) | 222 (51.6%) | 0.982 (0.779–1.237) | 0.867 (0.633–1.188) |
| Variable | GAS5 (rs145204276) | |||
|---|---|---|---|---|
| Ins/Ins (%) (n = 191) | Ins/Del + Del/Del (%) (n = 239) | OR (95% CI) | p Value | |
| Stage | ||||
| Non-muscle-invasive tumor | 104 (54.5%) | 131 (54.8%) | 1.000 (reference) | |
| Muscle-invasive tumor | 87 (45.5%) | 108 (45.2%) | 0.986 (0.673–1.444) | 0.940 |
| Tumor T status | ||||
| Ta | 44 (23.0%) | 46 (19.3%) | 1.000 (reference) | |
| T1-T2 | 81 (42.4%) | 116 (48.5%) | 1.370 (0.830–2.262) | 0.218 |
| T3-T4 | 66 (34.6%) | 77 (32.2%) | 1.116 (0.658–1.892) | 0.684 |
| Lymph node status | ||||
| N0 | 166 (86.9%) | 213 (89.1%) | 1.000 (reference) | |
| N1 + N2 | 25 (13.1%) | 26 (10.9%) | 0.811 (0.451–1.455) | 0.481 |
| Metastasis | ||||
| M0 | 186 (97.4%) | 230 (96.2%) | 1.000 (reference) | |
| M1 | 5 (2.6%) | 9 (3.8%) | 1.456 (0.480–4.418) | 0.505 |
| Histopathologic grading | ||||
| Low grade | 23 (12.0%) | 30 (12.6%) | 1.000 (reference) | |
| High grade | 168 (88.0%) | 209 (87.4%) | 0.954 (0.534–1.703) | 0.873 |
| Variable | GAS5 (rs145204276) | |||
|---|---|---|---|---|
| Ins/Ins (%) (n = 68) | Ins/Del + Del/Del (%) (n = 91) | OR (95% CI) | p Value | |
| Stage | ||||
| Non muscle invasive tumor | 38 (55.9%) | 46 (50.5%) | 1.000 (reference) | |
| Muscle invasive tumor | 30 (44.1%) | 45 (49.5%) | 1.239 (0.659–2.329) | 0.505 |
| Tumor T status | ||||
| Ta | 17 (25.0%) | 9 (9.9%) | 1.000 (reference) | |
| T1-T2 | 28 (41.2%) | 50 (54.9%) | 3.373 (1.329–8.558) | 0.009 * |
| T3-T4 | 23 (33.8%) | 32 (35.2%) | 2.628 (1.001–6.929) | 0.048 * |
| Lymph node status | ||||
| N0 | 60 (88.2%) | 81 (89.0%) | 1.000 (reference) | |
| N1 + N2 | 8 (11.8%) | 10 (11.0%) | 0.926 (0.345–2.486) | 0.879 |
| Metastasis | ||||
| M0 | 68 (100%) | 88 (96.7%) | 1.000 (reference) | |
| M1 | 0 (0.0%) | 3 (3.3%) | 0.131 | |
| Histopathologic grading | ||||
| Low grade | 9 (13.2%) | 5 (5.5%) | 1.000 (reference) | |
| High grade | 59 (86.8%) | 86 (94.5%) | 2.624 (0.837–8.223) | 0.088 |
| Variable | Hazard Ratio (95% Confidence Interval) | p-Value |
|---|---|---|
| Tumor T status (T1 + T2 vs. T3 + T4) | 3.355 (1.604–7.015) | <0.001 |
| GAS5 expression (low vs. high) | 0.491 (0.271–0.888) | =0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, W.-C.; Chen, C.-J.; Chen, P.-N.; Wang, S.-S.; Hsieh, M.-J.; Yang, S.-F. Impact of Gene Polymorphisms in GAS5 on Urothelial Cell Carcinoma Development and Clinical Characteristics. Diagnostics 2020, 10, 260. https://doi.org/10.3390/diagnostics10050260
Weng W-C, Chen C-J, Chen P-N, Wang S-S, Hsieh M-J, Yang S-F. Impact of Gene Polymorphisms in GAS5 on Urothelial Cell Carcinoma Development and Clinical Characteristics. Diagnostics. 2020; 10(5):260. https://doi.org/10.3390/diagnostics10050260
Chicago/Turabian StyleWeng, Wei-Chun, Chih-Jung Chen, Pei-Ni Chen, Shian-Shiang Wang, Ming-Ju Hsieh, and Shun-Fa Yang. 2020. "Impact of Gene Polymorphisms in GAS5 on Urothelial Cell Carcinoma Development and Clinical Characteristics" Diagnostics 10, no. 5: 260. https://doi.org/10.3390/diagnostics10050260
APA StyleWeng, W.-C., Chen, C.-J., Chen, P.-N., Wang, S.-S., Hsieh, M.-J., & Yang, S.-F. (2020). Impact of Gene Polymorphisms in GAS5 on Urothelial Cell Carcinoma Development and Clinical Characteristics. Diagnostics, 10(5), 260. https://doi.org/10.3390/diagnostics10050260







