Circulating Tumor Cells Enumerated by a Centrifugal Microfluidic Device as a Predictive Marker for Monitoring Ovarian Cancer Treatment: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. FAST Disc Enumeration of CTCs
2.3. Cell Culture and Spike Experiment
2.4. Statistical Analysis
3. Results
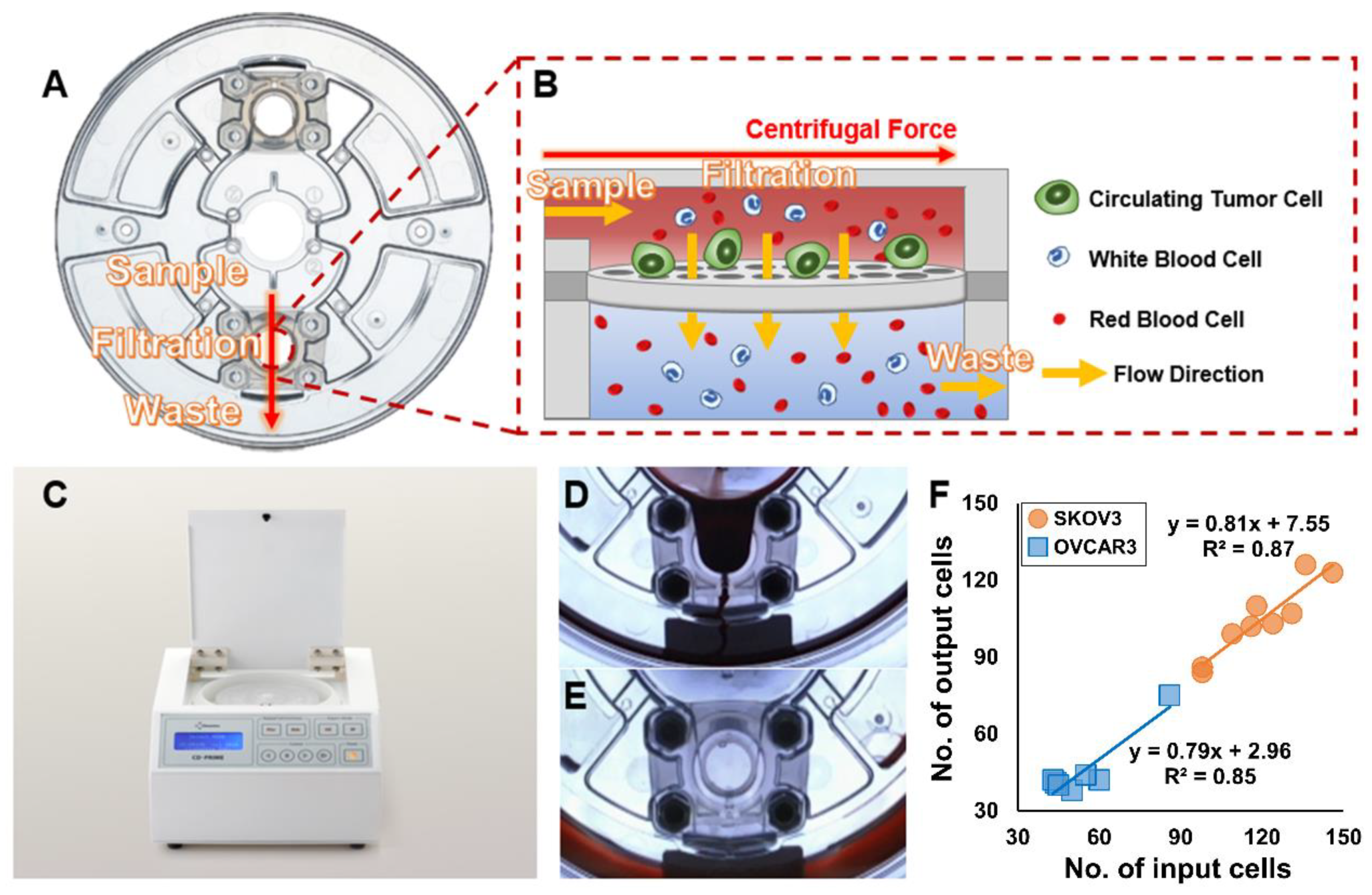
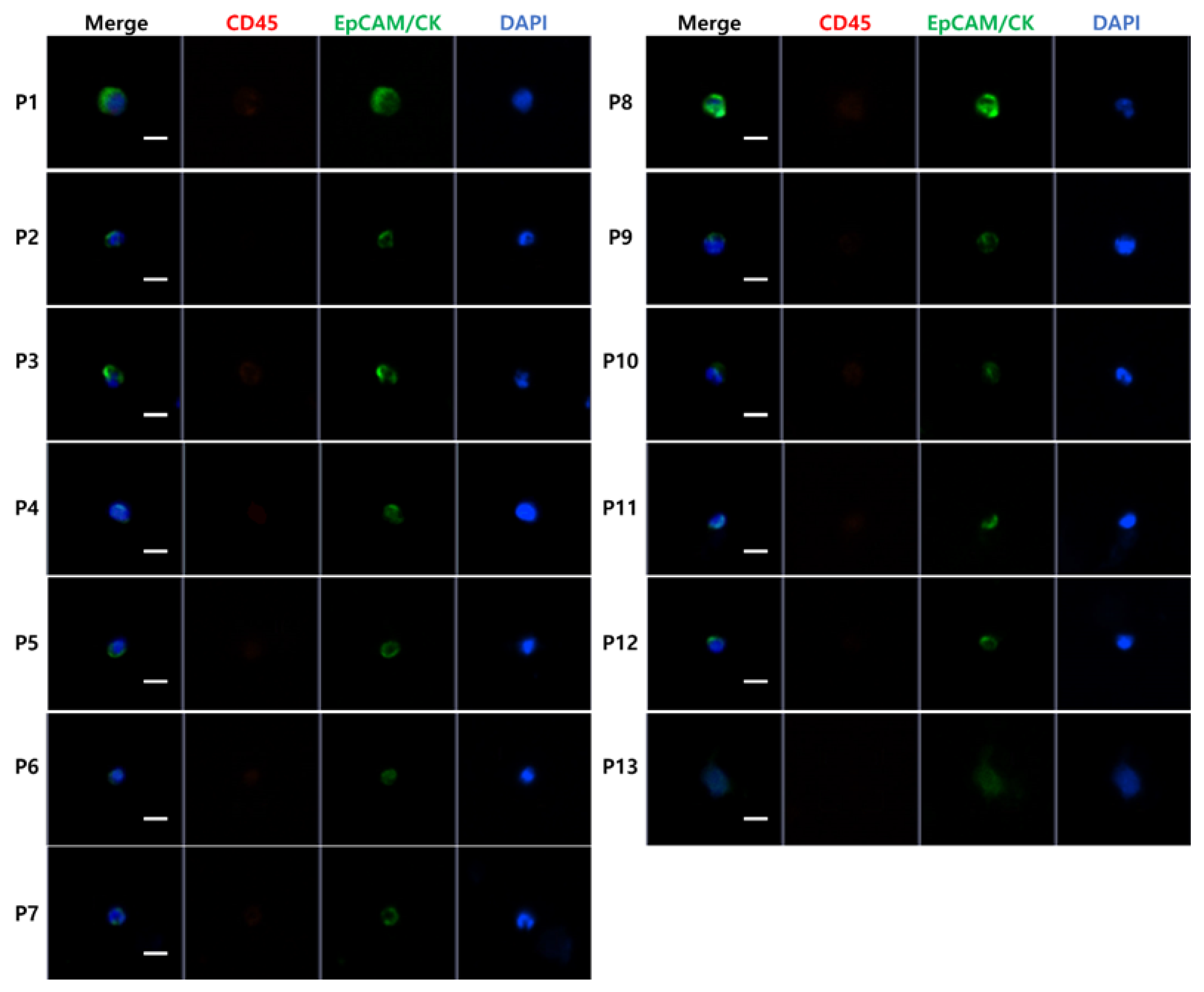
3.1. High-Throughput, Efficient, Label-Free Isolation of CTCs from Whole Blood Using the FAST Disc
3.2. Patient Characteristics
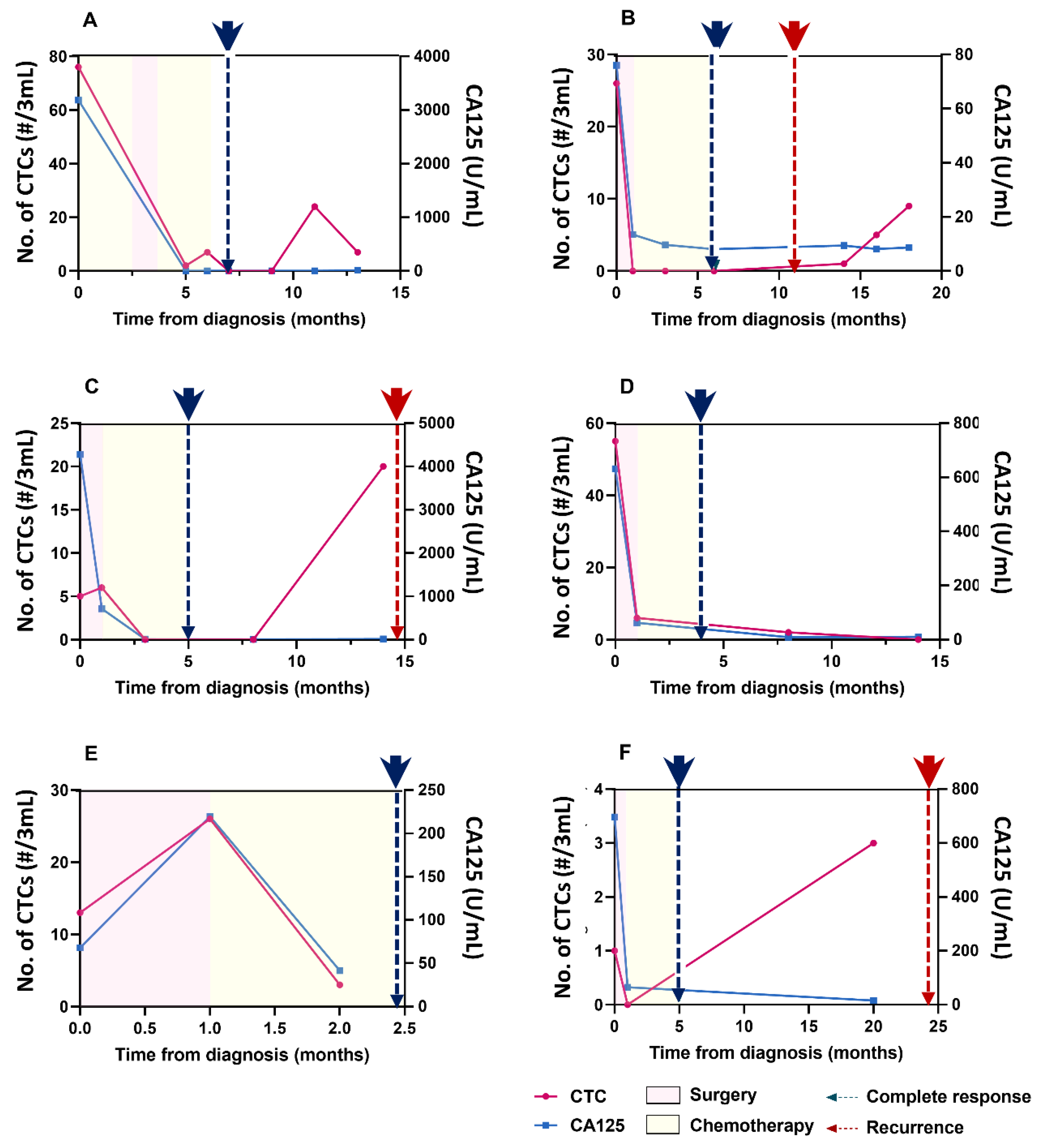
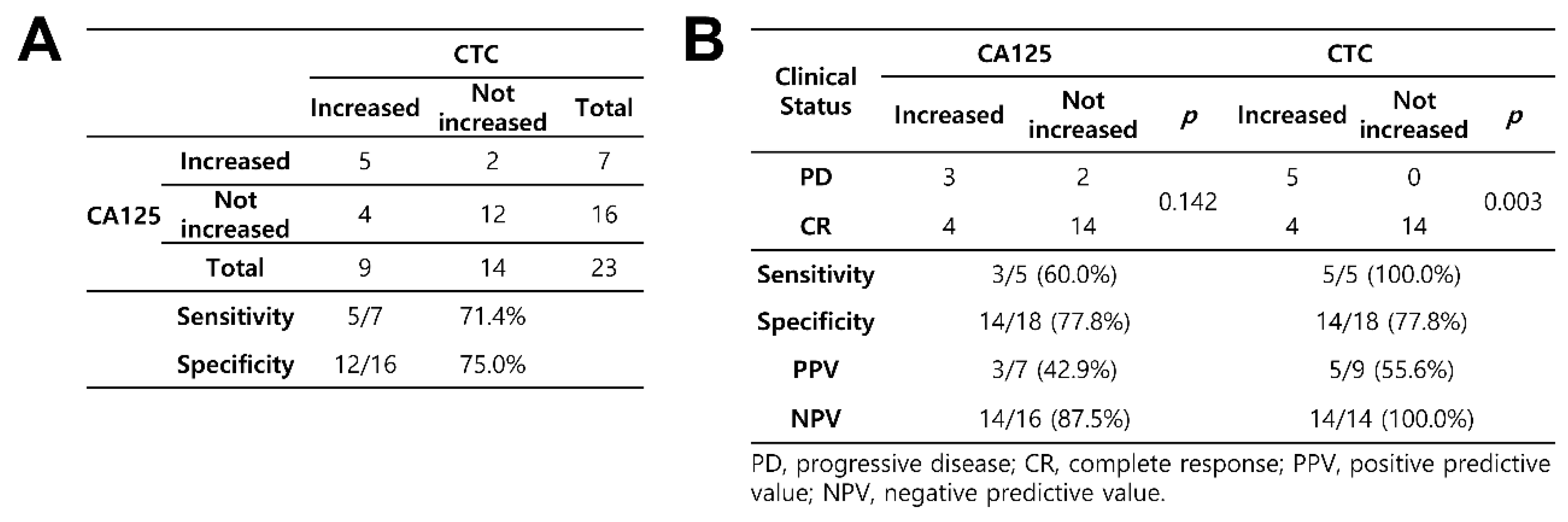
3.3. Detection of CTCs and Their Correlation with CA125 Concentrations
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baldwin, L.A.; Huang, B.; Miller, R.W.; Tucker, T.; Goodrich, S.T.; Podzielinski, I.; DeSimone, C.P.; Ueland, F.R.; van Nagell, J.R.; Seamon, L.G. Ten-year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012, 120, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.; Bast, R.C., Jr.; Kelloff, G.J.; Barrett, J.C.; Carter, S.K.; Nisen, P.D.; Sigman, C.C.; Parkinson, D.R.; Ruddon, R.W. Use of CA-125 in clinical trial evaluation of new therapeutic drugs for ovarian cancer. Clin. Cancer Res. 2004, 10, 3919–3926. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Hollingworth, J.; Reynolds, T.M. The role of CA125 in clinical practice. J. Clin. Pathol. 2005, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Buamah, P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265. [Google Scholar] [CrossRef]
- Masuda, T.; Hayashi, N.; Iguchi, T.; Ito, S.; Eguchi, H.; Mimori, K. Clinical and biological significance of circulating tumor cells in cancer. Mol. Oncol. 2016, 10, 408–417. [Google Scholar] [CrossRef]
- van Dalum, G.; Stam, G.J.; Scholten, L.F.; Mastboom, W.J.; Vermes, I.; Tibbe, A.G.; De Groot, M.R.; Terstappen, L.W. Importance of circulating tumor cells in newly diagnosed colorectal cancer. Int. J. Oncol. 2015, 46, 1361–1368. [Google Scholar] [CrossRef]
- Janni, W.J.; Rack, B.; Terstappen, L.W.; Pierga, J.Y.; Taran, F.A.; Fehm, T.; Hall, C.; de Groot, M.R.; Bidard, F.C.; Friedl, T.W.; et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin. Cancer Res. 2016, 22, 2583–2593. [Google Scholar] [CrossRef]
- Giannopoulou, L.; Kasimir-Bauer, S.; Lianidou, E.S. Liquid biopsy in ovarian cancer: Recent advances on circulating tumor cells and circulating tumor DNA. Clin. Chem. Lab. Med. 2018, 56, 186–197. [Google Scholar] [CrossRef]
- Judson, P.L.; Geller, M.A.; Bliss, R.L.; Boente, M.P.; Downs, L.S., Jr.; Argenta, P.A.; Carson, L.F. Preoperative detection of peripherally circulating cancer cells and its prognostic significance in ovarian cancer. Gynecol. Oncol. 2003, 91, 389–394. [Google Scholar] [CrossRef]
- Fan, T.; Zhao, Q.; Chen, J.J.; Chen, W.T.; Pearl, M.L. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol. Oncol. 2009, 112, 185–191. [Google Scholar] [CrossRef]
- Pearl, M.L.; Dong, H.; Tulley, S.; Zhao, Q.; Golightly, M.; Zucker, S.; Chen, W.T. Treatment monitoring of patients with epithelial ovarian cancer using invasive circulating tumor cells (iCTCs). Gynecol. Oncol. 2015, 137, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Doyle, G.V.; Terstappen, L.W. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J. Oncol. 2010, 2010, 617421. [Google Scholar] [CrossRef]
- Poveda, A.; Kaye, S.B.; McCormack, R.; Wang, S.; Parekh, T.; Ricci, D.; Lebedinsky, C.A.; Tercero, J.C.; Zintl, P.; Monk, B.J. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol. Oncol. 2011, 122, 567–572. [Google Scholar] [CrossRef]
- Behbakht, K.; Sill, M.W.; Darcy, K.M.; Rubin, S.C.; Mannel, R.S.; Waggoner, S.; Schilder, R.J.; Cai, K.Q.; Godwin, A.K.; Alpaugh, R.K. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: A Gynecologic Oncology Group study. Gynecol. Oncol. 2011, 123, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef]
- Lampignano, R.; Yang, L.; Neumann, M.H.D.; Franken, A.; Fehm, T.; Niederacher, D.; Neubauer, H. A Novel Workflow to Enrich and Isolate Patient-Matched EpCAM(high) and EpCAM(low/negative) CTCs Enables the Comparative Characterization of the PIK3CA Status in Metastatic Breast Cancer. Int. J. Mol. Sci. 2017, 18, 1885. [Google Scholar] [CrossRef]
- Pearl, M.L.; Zhao, Q.; Yang, J.; Dong, H.; Tulley, S.; Zhang, Q.; Golightly, M.; Zucker, S.; Chen, W.T. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 581–590. [Google Scholar] [CrossRef]
- Vitale, S.G.; Capriglione, S.; Zito, G.; Lopez, S.; Gulino, F.A.; Di Guardo, F.; Vitagliano, A.; Noventa, M.; La Rosa, V.L.; Sapia, F.; et al. Management of endometrial, ovarian and cervical cancer in the elderly: Current approach to a challenging condition. Arch. Gynecol. 2019, 299, 299–315. [Google Scholar] [CrossRef]
- Schuurman, M.; Kruitwagen, R.; Portielje, J.; Roes, E.; Lemmens, V.; Van der Aa, M. Treatment and outcome of elderly patients with advanced stage ovarian cancer: A nationwide analysis. Gynecol. Oncol. 2018, 149, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Men, X.; Zhang, W.; Lei, P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J. Cancer 2014, 51, 72. [Google Scholar]
- Valenti, G.; Vitale, S.G.; Tropea, A.; Biondi, A.; Laganà, A.S. Tumor markers of uterine cervical cancer: A new scenario to guide surgical practice? Updates Surg. 2017, 69, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Peedicayil, J. The role of DNA methylation in the pathogenesis and treatment of cancer. Curr. Clin. Pharmacol. 2012, 7, 333–340. [Google Scholar] [CrossRef]
- Jazaeri, A.A. Molecular profiles of hereditary epithelial ovarian cancers and their implications for the biology of this disease. Mol. Oncol. 2009, 3, 151–156. [Google Scholar] [CrossRef]
- Tessitore, A.; Gaggiano, A.; Cicciarelli, G.; Verzella, D.; Capece, D.; Fischietti, M.; Zazzeroni, F.; Alesse, E. Serum biomarkers identification by mass spectrometry in high-mortality tumors. Int. J. Proteom. 2013, 2013, 125858. [Google Scholar] [CrossRef]
- Tang, L.; Yang, J.; Ng, S.K.; Rodriguez, N.; Choi, P.W.; Vitonis, A.; Wang, K.; McLachlan, G.J.; Caiazzo, R.J., Jr.; Liu, B.C.; et al. Autoantibody profiling to identify biomarkers of key pathogenic pathways in mucinous ovarian cancer. Eur. J. Cancer 2010, 46, 170–179. [Google Scholar] [CrossRef]
- Tringler, B.; Liu, W.; Corral, L.; Torkko, K.C.; Enomoto, T.; Davidson, S.; Lucia, M.S.; Heinz, D.E.; Papkoff, J.; Shroyer, K.R. B7-H4 overexpression in ovarian tumors. Gynecol. Oncol. 2006, 100, 44–52. [Google Scholar] [CrossRef]
- Wang, P.; Wu, X.; Chen, W.; Liu, J.; Wang, X. The lysophosphatidic acid (LPA) receptors their expression and significance in epithelial ovarian neoplasms. Gynecol. Oncol. 2007, 104, 714–720. [Google Scholar] [CrossRef]
- Kim, T.H.; Lim, M.; Park, J.; Oh, J.M.; Kim, H.; Jeong, H.; Lee, S.J.; Park, H.C.; Jung, S.; Kim, B.C.; et al. FAST: Size-Selective, Clog-Free Isolation of Rare Cancer Cells from Whole Blood at a Liquid-Liquid Interface. Anal. Chem. 2017, 89, 1155–1162. [Google Scholar] [CrossRef]
- Kang, H.M.; Kim, G.H.; Jeon, H.K.; Kim, D.H.; Jeon, T.Y.; Park, D.Y.; Jeong, H.; Chun, W.J.; Kim, M.H.; Park, J.; et al. Circulating tumor cells detected by lab-on-a-disc: Role in early diagnosis of gastric cancer. PLoS ONE 2017, 12, e0180251. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Kim, G.H.; I, H.; Park, S.J.; Lee, M.W.; Lee, B.E.; Park, D.Y.; Cho, Y.K. Circulating tumor cells detected using fluid-assisted separation technique in esophageal squamous cell carcinoma. J. Gastroenterol. Hepatol. 2019, 34, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.H.; Kim, G.H.; Song, G.A.; Han, I.S.; Park, E.Y.; Kim, H.S.; Jo, H.J.; Ko, S.H.; Park, D.Y.; Cho, Y.K. Clinical Potential of Circulating Tumor Cells in Colorectal Cancer: A Prospective Study. Clin. Transl. Gastroenterol. 2019, 10, e00055. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Park, J.; Lowe, A.C.; Jeong, H.-O.; Lee, S.; Park, H.C.; Lee, K.; Kim, G.H.; Kim, M.-H.; Cho, Y.-K. A lab-on-a-disc platform enables serial monitoring of individual CTCs associated with tumor progression during EGFR-targeted therapy for patients with NSCLC. Theranostics 2020, 10, 5181–5194. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, Z.; Smith, K.; Kuczler, M.; Reyes, D.; Amend, S.R.; Cho, Y.K.; Xue, W.; Pienta, K.J. The combination of size-based separation and selection-free technology provides higher circulating tumor cells detection sensitivity than either method alone in patients with metastatic prostate cancer. BJU Int. 2020. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, L.; Chen, Y.; Qing, C. Circulating cell-free DNA and circulating tumor cells, the “liquid biopsies” in ovarian cancer. J. Ovarian Res. 2017, 10, 75. [Google Scholar] [CrossRef]
- Brouwer, A.; De Laere, B.; Peeters, D.; Peeters, M.; Salgado, R.; Dirix, L.; Van Laere, S. Evaluation and consequences of heterogeneity in the circulating tumor cell compartment. Oncotarget 2016, 7, 48625–48643. [Google Scholar] [CrossRef]
- Van Berckelaer, C.; Brouwers, A.J.; Peeters, D.J.; Tjalma, W.; Trinh, X.B.; van Dam, P.A. Current and future role of circulating tumor cells in patients with epithelial ovarian cancer. Eur. J. Surg. Oncol. 2016, 42, 1772–1779. [Google Scholar] [CrossRef]
- Liu, J.F.; Kindelberger, D.; Doyle, C.; Lowe, A.; Barry, W.T.; Matulonis, U.A. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol. Oncol. 2013, 131, 352–356. [Google Scholar] [CrossRef]
- Obermayr, E.; Castillo-Tong, D.C.; Pils, D.; Speiser, P.; Braicu, I.; Van Gorp, T.; Mahner, S.; Sehouli, J.; Vergote, I.; Zeillinger, R. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance—A study of the OVCAD consortium. Gynecol. Oncol. 2013, 128, 15–21. [Google Scholar] [CrossRef]
- Kim, M.; Suh, D.H.; Choi, J.Y.; Bu, J.; Kang, Y.T.; Kim, K.; No, J.H.; Kim, Y.B.; Cho, Y.H. Post-debulking circulating tumor cell as a poor prognostic marker in advanced stage ovarian cancer: A prospective observational study. Medicine 2019, 98, e15354. [Google Scholar] [CrossRef] [PubMed]
| No. | Age at Diagnosis (years) | Initial FIGO Stage | Histology | Initial CA125 (U/mL) | Initial CTCs (/3 mL) | Treatment Response | CA125 after Therapy (U/mL) | CTCs after Therapy (/3 mL) | No. of Blood Samples |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | IIIC | High-grade serous carcinoma | 3186.5 | 76 | CR | 5.8 | 0 | 7 |
| 2 | 67 | IIIC | High-grade serous carcinoma | 76 | 26 | CR | 8.1 | 0 | 7 |
| 3 | 55 | IIIC | High-grade serous carcinoma | 4278.5 | 5 | CR | 11.3 | 0 | 5 |
| 4 | 53 | IC | Mucinous carcinoma | 18.2 | 64 | CR | 8.5 | 1 | 5 |
| 5 | 49 | IIIA2 | High-grade serous carcinoma | 631.9 | 55 | CR | 9.3 | 2 | 4 |
| 6 | 56 | IC | High-grade serous carcinoma | 25.7 | 0 | CR | 7.7 | 3 | 4 |
| 7 | 75 | IV | Adenocarcinoma with serous carcinoma | 10,000 | 0 | PR | 244.5 | 0 | 4 |
| 8 | 40 | IIB | Clear cell carcinoma | 34.6 | 13 | CR | 41.6 | 3 | 3 |
| 9 | 59 | IIB | High-grade serous carcinoma | 696.4 | 1 | CR | 65.1 | 0 | 3 |
| 10 | 46 | IIIC | Clear cell carcinoma | 2432.8 | 6 | CR | 153.1 | 0 | 2 |
| 11 | 47 | IV | High-grade serous carcinoma | 553.3 | 2 | CR | NA | NA | 1 |
| 12 | 66 | IIIC | High-grade serous carcinoma | 3399.8 | 10 | CR | NA | NA | 1 |
| 13 | 65 | IIIC | High-grade serous carcinoma | 8767.4 | 4 | PR | NA | NA | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Lim, M.; Kim, J.Y.; Shin, S.-J.; Cho, Y.-K.; Cho, C.H. Circulating Tumor Cells Enumerated by a Centrifugal Microfluidic Device as a Predictive Marker for Monitoring Ovarian Cancer Treatment: A Pilot Study. Diagnostics 2020, 10, 249. https://doi.org/10.3390/diagnostics10040249
Kim H, Lim M, Kim JY, Shin S-J, Cho Y-K, Cho CH. Circulating Tumor Cells Enumerated by a Centrifugal Microfluidic Device as a Predictive Marker for Monitoring Ovarian Cancer Treatment: A Pilot Study. Diagnostics. 2020; 10(4):249. https://doi.org/10.3390/diagnostics10040249
Chicago/Turabian StyleKim, Hyera, Minji Lim, Jin Young Kim, So-Jin Shin, Yoon-Kyoung Cho, and Chi Heum Cho. 2020. "Circulating Tumor Cells Enumerated by a Centrifugal Microfluidic Device as a Predictive Marker for Monitoring Ovarian Cancer Treatment: A Pilot Study" Diagnostics 10, no. 4: 249. https://doi.org/10.3390/diagnostics10040249
APA StyleKim, H., Lim, M., Kim, J. Y., Shin, S.-J., Cho, Y.-K., & Cho, C. H. (2020). Circulating Tumor Cells Enumerated by a Centrifugal Microfluidic Device as a Predictive Marker for Monitoring Ovarian Cancer Treatment: A Pilot Study. Diagnostics, 10(4), 249. https://doi.org/10.3390/diagnostics10040249





