Geographical Accessibility to Glucose-6-Phosphate Dioxygenase Deficiency Point-of-Care Testing for Antenatal Care in Ghana
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Data Collection
2.3. Variables and Operational Definitions
2.3.1. Availability
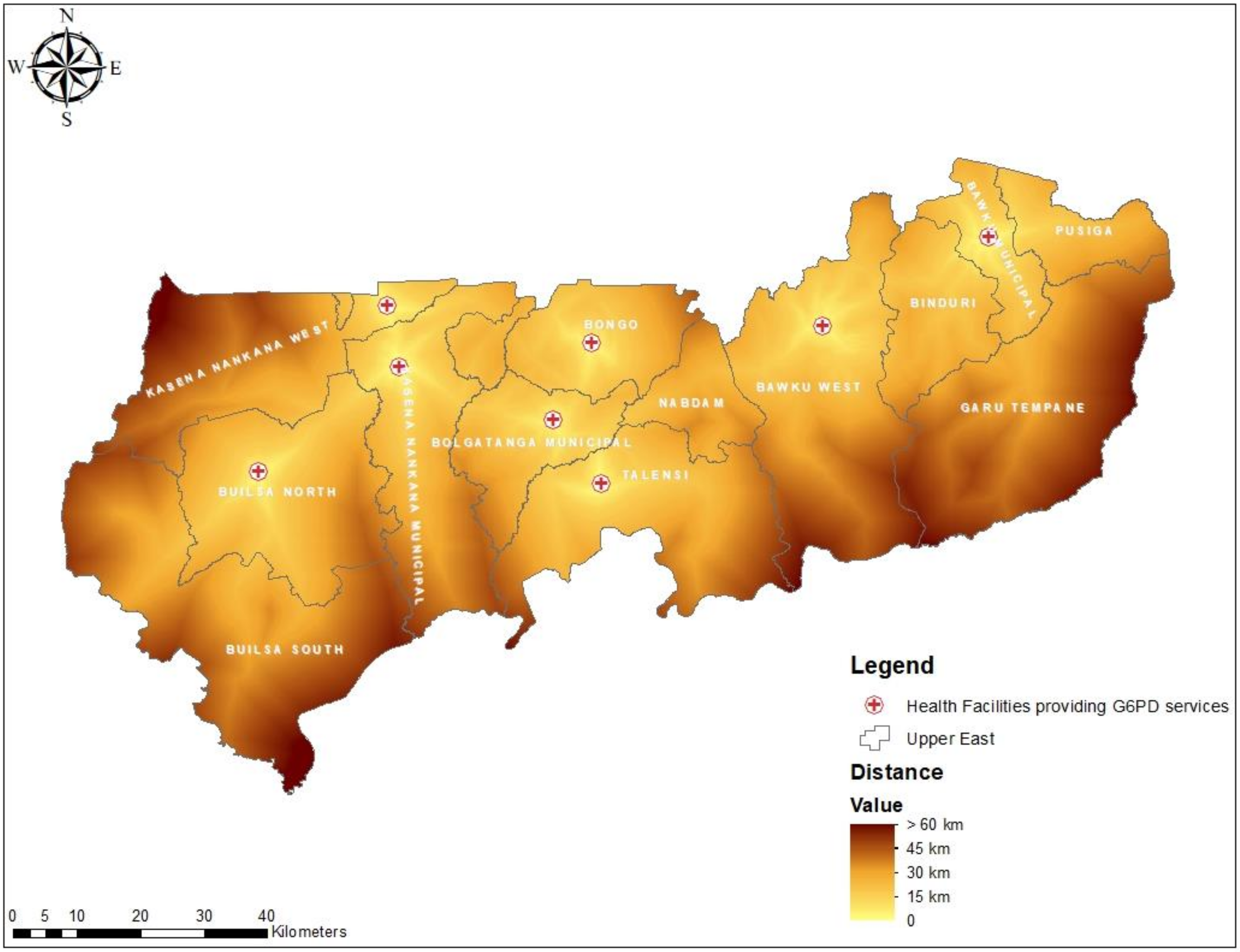
2.3.2. Distance
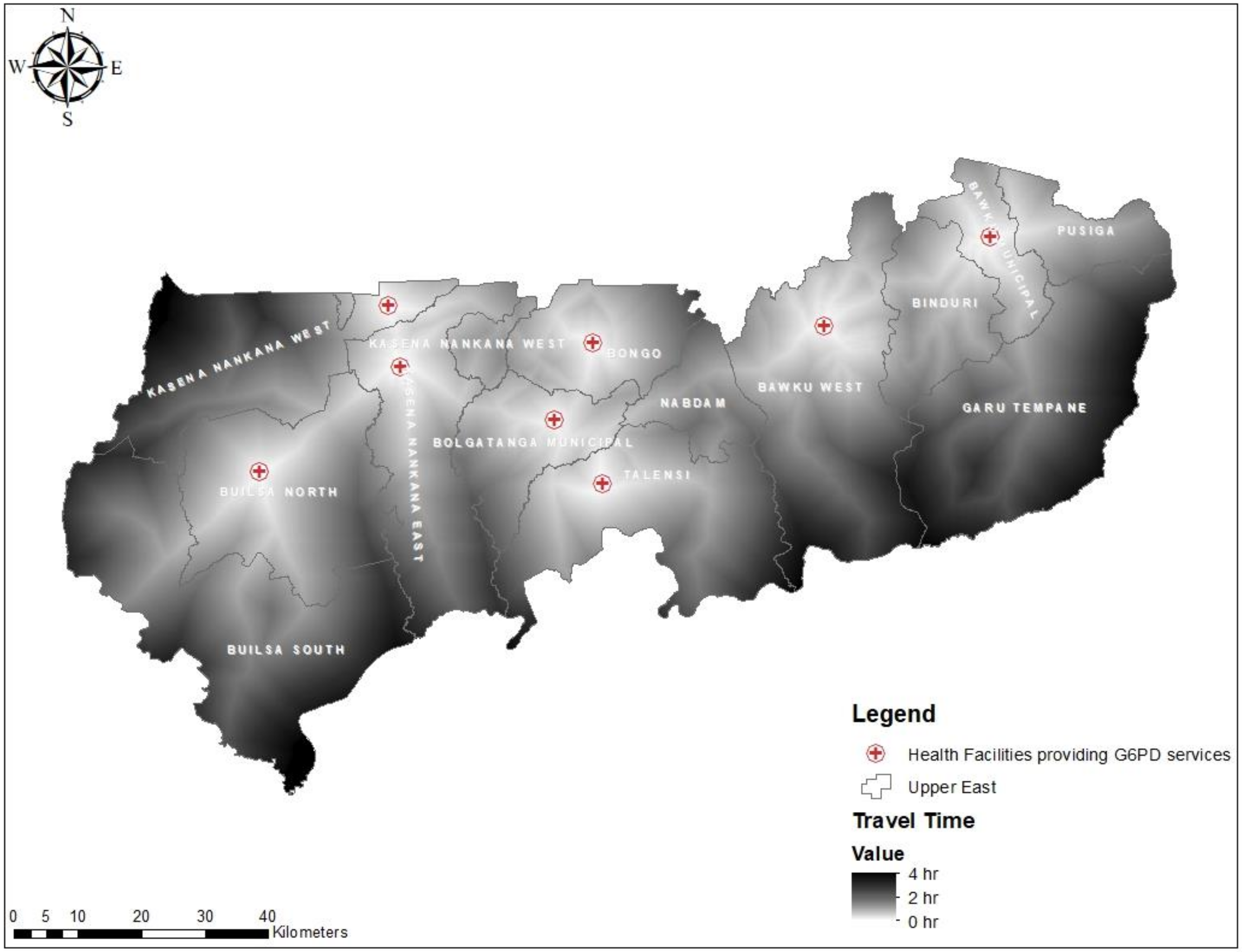
2.3.3. Travel Time
2.4. Data Analysis and Mapping
2.5. Ethics Approval
3. Results
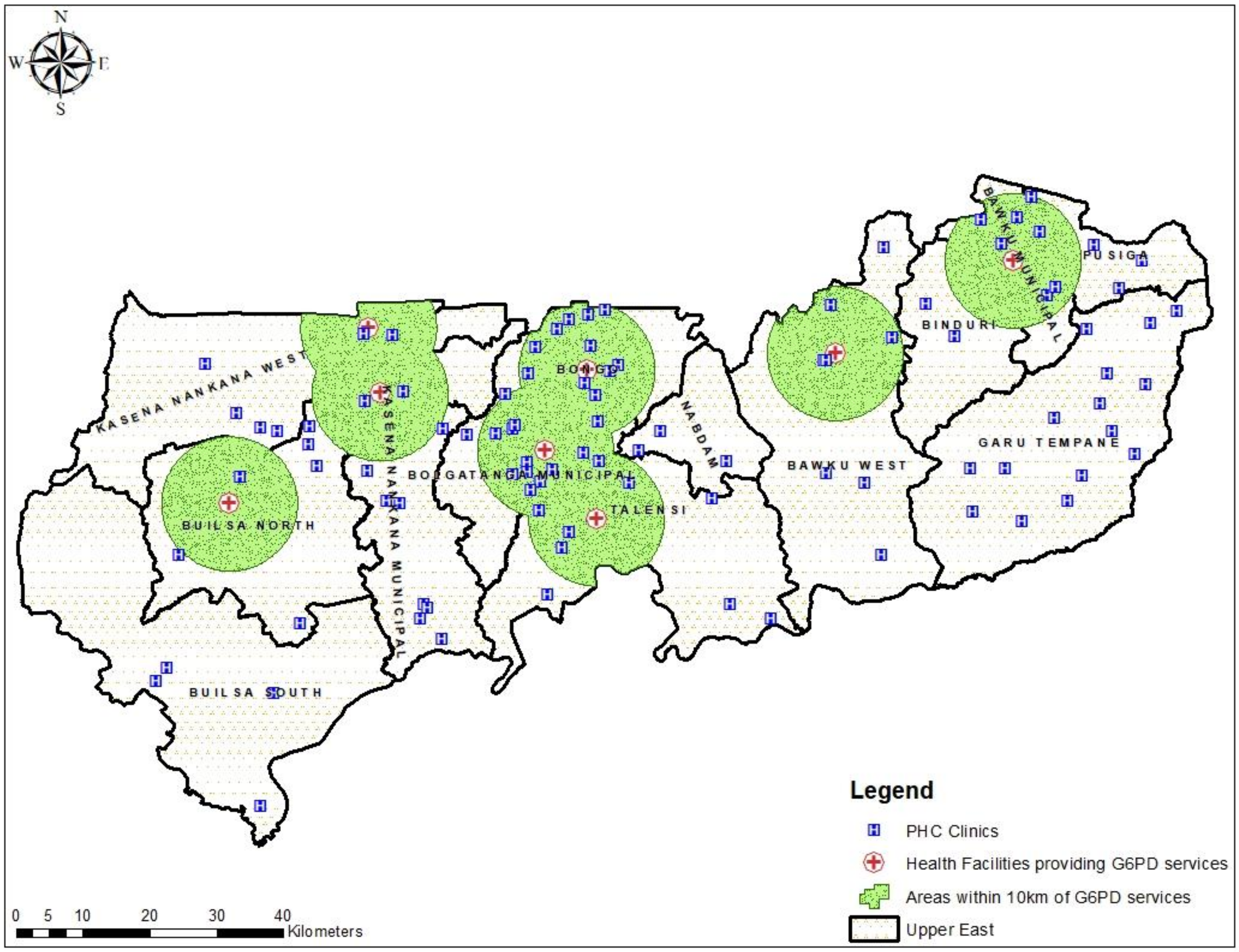
3.1. Characteristics of the Participated PHC Clinics in the Study
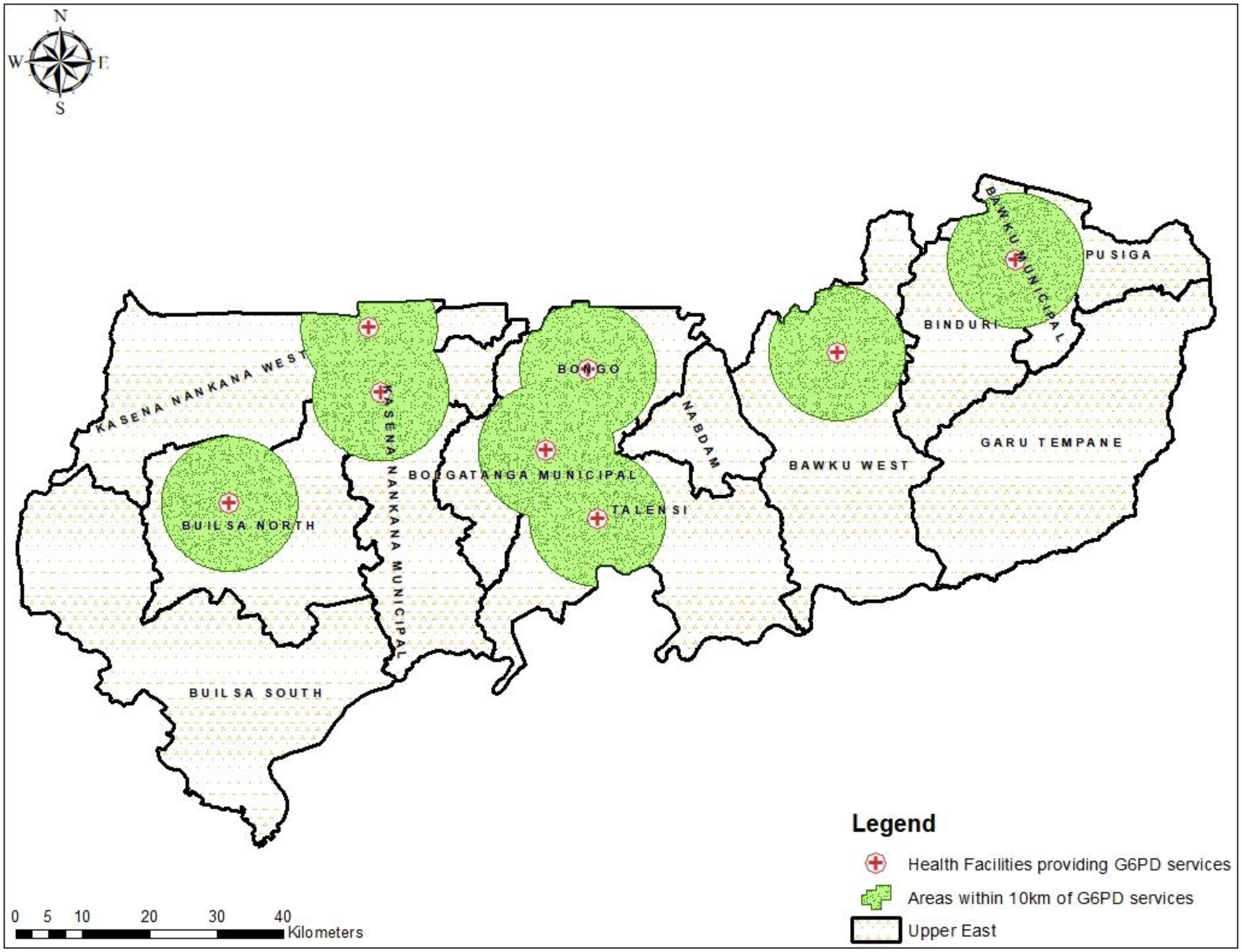
3.2. Geographical Distribution of Public Health Facilities Providing G6PD Deficiency Testing in UER
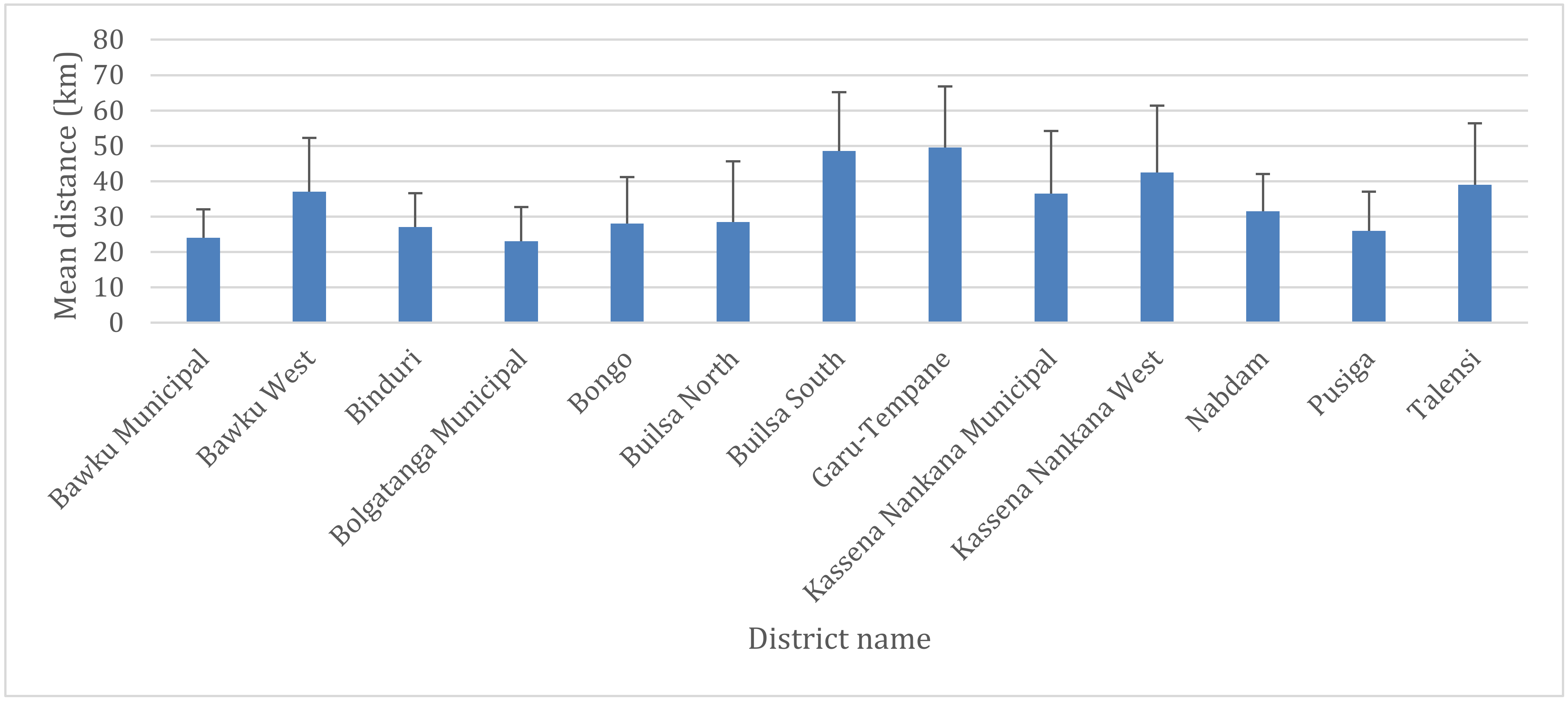
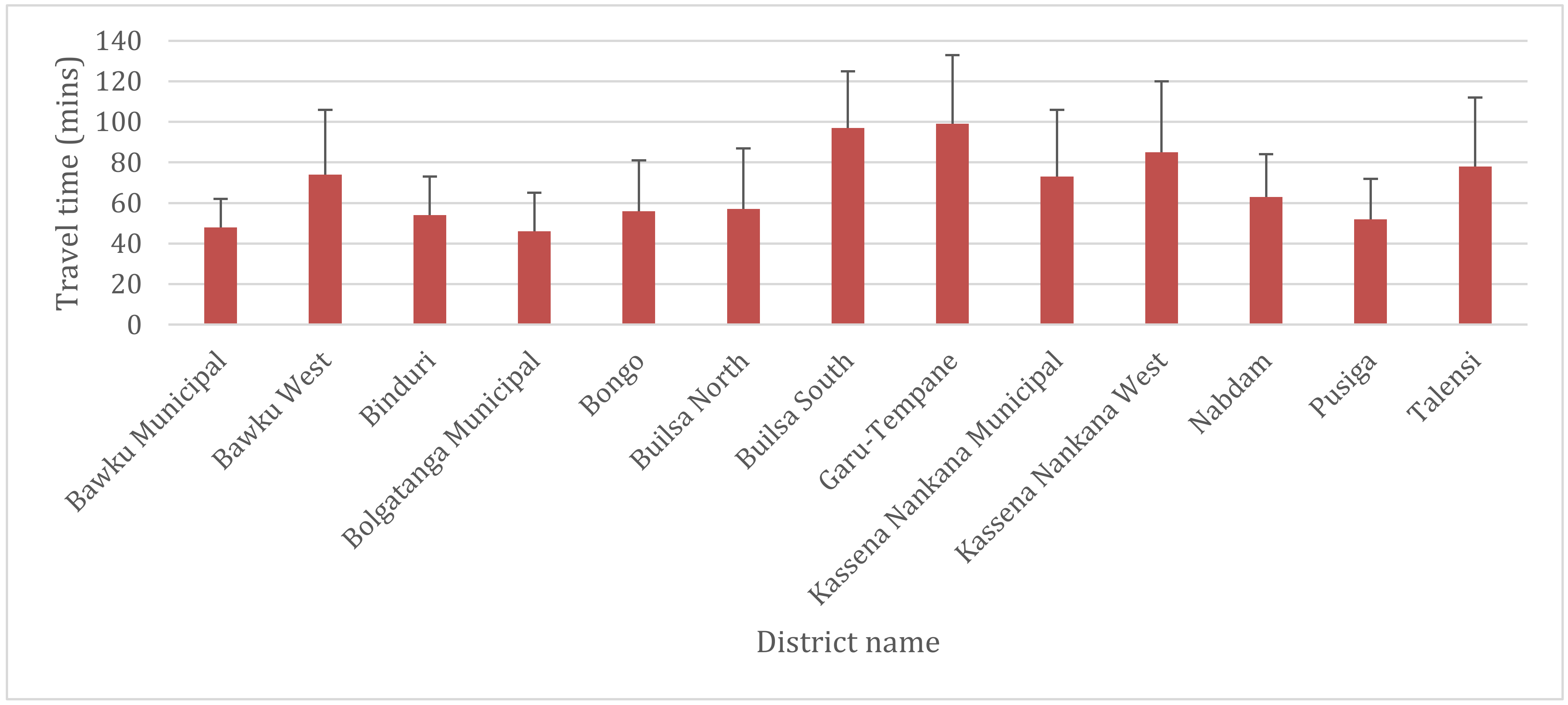
3.3. Geographical Access to Health Facilities for G6PD Deficiency POC Testing in the UER
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANC | Antenatal care |
| COVID-19 | Coronavirus Disease 2019 |
| G6PD | Glucose-6-Phosphate Dehydrogenase |
| GIS | Geographic Information Systems |
| PHC | Primary Healthcare |
| POC | Point-of-Care |
| RDT | Rapid Diagnostic Test |
| SDGs | Sustainable Development Goals |
| UER | Upper East Region |
| WHO | World Health Organization |
References
- Lee, J.; Kim, T.I.; Kang, J.-M.; Jun, H.; Lê, H.G.; Thái, T.L.; Sohn, W.-M.; Myint, M.K.; Lin, K.; Kim, T.-S.; et al. Prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency among malaria patients in Upper Myanmar. BMC Infect. Dis. 2018, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Glucose-6-Phosphate Dehydrogenase Deficiency. N. Engl. J. Med. 1991, 324, 169–174. [Google Scholar] [PubMed]
- Beutler, E. G6PD deficiency. Blood 1994, 84, 3613–3636. [Google Scholar] [CrossRef] [PubMed]
- Nkhoma, E.T.; Poole, C.; Vannappagari, V.; Hall, S.A.; Beutler, E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. Blood Cells Mol. Dis. 2009, 42, 267–278. [Google Scholar] [CrossRef]
- Adu-Gyasi, D.; Asante, K.P.; Newton, S.; Dosoo, D.; Amoako, S.; Adjei, G.; Amoako, N.; Ankrah, L.; Tchum, S.K.; Mahama, E.; et al. Evaluation of the diagnostic accuracy of CareStart G6PD deficiency rapid diagnostic test (RDT) in a malaria endemic area in Ghana, Africa. PLoS ONE 2015, 10, e0125796. [Google Scholar] [CrossRef]
- Hwang, S.; Mruk, K.; Rahighi, S.; Raub, A.G.; Chen, C.-H.; Dorn, L.E.; Horikoshi, N.; Wakatsuki, S.; Chen, J.; Mochly-Rosen, D. Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat. Commun. 2018, 9, 4045. [Google Scholar] [CrossRef]
- Luzzatto, L.; Seneca, E. G6PD deficiency: A classic example of pharmacogenetics with on-going clinical implications. Br. J. Haematol. 2013, 164, 469–480. [Google Scholar] [CrossRef]
- Baird, J.K. Origins and implications of neglect of G6PD deficiency and primaquine toxicity inPlasmodium vivaxmalaria. Pathog. Glob. Health 2015, 109, 93–106. [Google Scholar] [CrossRef]
- Vick, J.D. Chloroquine Is Not a Harmless Panacea for COVID-19—There’s a Real Safety Concern with Malaria Drug USA: Medpagetoday. 2020. Available online: https://www.medpagetoday.com/infectiousdisease/covid19/85552 (accessed on 5 April 2020).
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Diallo, N.; Akweongo, P.; Maya, E.T.; Aikins, M.; Sarfo, B. Burden of malaria in mobile populations in the Greater Accra region, Ghana: A cross-sectional study. Malar. J. 2017, 16, 109. [Google Scholar] [CrossRef]
- Owusu-Ofori, A.; Gadzo, D.; Bates, I. Transfusion-transmitted malaria: Donor prevalence of parasitaemia and a survey of healthcare workers knowledge and practices in a district hospital in Ghana. Malar. J. 2016, 15, 234. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Malaria Geneva: World Health Organisation; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/malaria/en/ (accessed on 4 August 2019).
- World Health Organisation. World Malaria Report 2018 Geneva: World Health Organisation; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf (accessed on 4 August 2019).
- National Malaria Control Programme. Intermittent Preventive Treatment in Pregnancy (IPTp) Accra: Ghana Health Service. 2019. Available online: http://www.ghanahealthservice.org/malaria/item.php?nmcpiid=60&nmcpscid=114&nmcpcid=85 (accessed on 4 August 2019).
- Van Eijk, A.M.; A Larsen, D.; Kayentao, K.; Koshy, G.; Slaughter, D.E.C.; Roper, C.; Okell, L.C.; Desai, M.; Gutman, J.; Khairallah, C.; et al. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: A systematic review and meta-analysis. Lancet Infect. Dis. 2019, 19, 546–556. [Google Scholar] [CrossRef]
- Kajubi, R.; Ochieng, T.; Kakuru, A.; Jagannathan, P.; Nakalembe, M.; Ruel, T.; Opira, B.; Ochokoru, H.; Ategeka, J.; Nayebare, P.; et al. Monthly sulfadoxine-pyrimethamine versus dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: A double-blind, randomised, controlled, superiority trial. Lancet 2019, 393, 1428–1439. [Google Scholar] [CrossRef]
- Shulman, C.E.; Dorman, E.K.; Cutts, F.; Kawuondo, K.; Bulmer, J.N.; Peshu, N.; Marsh, K. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: A randomised placebo-controlled trial. Lancet 1999, 353, 632–636. [Google Scholar] [CrossRef]
- Schultz, L.J.; Steketee, R.W.; Macheso, A.; Kazembe, P.; Chitsulo, L.; Wirima, J.J. The Efficacy of Antimalarial Regimens Containing Sulfadoxine-Pyrimethamine and/or Chloroquine in Preventing Peripheral and Placental Plasmodium falciparum Infection among Pregnant Women in Malawi. Am. J. Trop. Med. Hyg. 1994, 51, 515–522. [Google Scholar] [CrossRef]
- Wilson, N.; Ceesay, F.K.; Obed, S.A.; Adjei, A.A.; Gyasi, R.K.; Rodney, P.; Ndjakani, Y.; Anderson, W.A.; Lucchi, N.W.; Stiles, J.K. Intermittent Preventive Treatment with Sulfadoxine-Pyrimethamine against Malaria and Anemia in Pregnant Women. Am. J. Trop. Med. Hyg. 2011, 85, 12–21. [Google Scholar] [CrossRef]
- World Health Organisation. Single Dose Primaquine as a Gametocytocide in Plasmodium Falciparum Malaria Geneva: World Health Organisation; WHO: Geneva, Switzerland, 2012; Available online: https://www.who.int/malaria/pq_updated_policy_recommendation_en_102012.pdf (accessed on 8 August 2019).
- Republic of Ghana. Anti-Malaria Drug Policy for Ghana Accra: Ministry of Health. 2009. Available online: https://www.ghanahealthservice.org/downloads/GHS_Antimalaria_drug_policy.pdf (accessed on 4 August 2019).
- White, N.J.; Li, G.; Gao, Q.; Luzzatto, L. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar. J. 2012, 11, 418. [Google Scholar] [CrossRef]
- Chan, T.K.; Todd, D.; Tso, S.C. Drug-induced haemolysis in glucose-6-phosphate dehydrogenase deficiency. BMJ 1976, 2, 1227–1229. [Google Scholar] [CrossRef][Green Version]
- Owusu, R.; Asante, K.P.; Mahama, E.; Awini, E.; Anyorigiya, T.; Dosoo, D.; Amu, A.; Jakpa, G.; Ofei, E.; Segbaya, S.; et al. Glucose-6-Phosphate Dehydrogenase Deficiency and Haemoglobin Drop after Sulphadoxine-Pyrimethamine Use for Intermittent Preventive Treatment of Malaria during Pregnancy in Ghana—A Cohort Study. PLoS ONE 2015, 10, 0136828. [Google Scholar] [CrossRef]
- Carter, N.; Pamba, A.; Duparc, S.; Waitumbi, J. Frequency of glucose-6-phosphate dehydrogenase deficiency in malaria patients from six African countries enrolled in two randomized anti-malarial clinical trials. Malar. J. 2011, 10, 241. [Google Scholar] [CrossRef]
- Owusu-Agyei, S.; Nettey, O.E.A.; Zandoh, C.; Sulemana, A.; Adda, R.; Amenga-Etego, S.; Mbacke, C. Demographic patterns and trends in Central Ghana: Baseline indicators from the Kintampo Health and Demographic Surveillance System. Glob. Health Action 2012, 5, 19033. [Google Scholar] [CrossRef] [PubMed]
- Amoako, N.; Asante, K.P.; Adjei, G.; Awandare, G.A.; Bimi, L.; Owusu-Agyei, S. Associations between Red Cell Polymorphisms and Plasmodium falciparum Infection in the Middle Belt of Ghana. PLoS ONE 2014, 9, e112868. [Google Scholar] [CrossRef] [PubMed]
- Kuupiel, D.; Tlou, B.; Bawontuo, V.; Mashamba-Thompson, T.P. Accessibility of pregnancy-related point-of-care diagnostic tests for maternal healthcare in rural primary healthcare facilities in Northern Ghana: A cross-sectional survey. Heliyon 2019, 5, e01236. [Google Scholar] [CrossRef] [PubMed]
- Kuupiel, D.; Tlou, B.; Bawontuo, V.; Drain, P.K.; Mashamba-Thompson, T.P. Poor supply chain management and stock-outs of point-of-care diagnostic tests in Upper East Region’s primary healthcare clinics, Ghana. PLoS ONE 2019, 14, e0211498. [Google Scholar] [CrossRef] [PubMed]
- Kuupiel, D.; Bawontuo, V.; Donkoh, A.; Drain, P.K.; Mashamba-Thompson, T.P. Empirical Framework for Point-of-Care Diagnostics Supply Chain Management for Accessibility and Sustainability of Diagnostic Services in Ghana’s Primary Health Care Clinics. Point Care J. Near Patient Test. Technol. 2019, 18, 72–75. [Google Scholar] [CrossRef]
- Kuupiel, D.; Adu, K.M.; Bawontuo, V.; Mashamba-Thompson, T.P. Geographical Accessibility to District Hospitals/Medical Laboratories for Comprehensive Antenatal Point-of-Care Diagnostic Services in the Upper East Region, Ghana. EClinicalMedicine 2019, 13, 74–80. [Google Scholar] [CrossRef]
- Ghana Statistical Service. 2010 Population & Housing Census: Summary Report of Final Results Accra: Ghana Statistical Service; 2012. Available online: http://www.statsghana.gov.gh/docfiles/2010phc/Census2010_Summary_report_of_final_results.pdf (accessed on 25 January 2020).
- Kuupiel, D.; Adu, K.; Apiribu, F.; Bawontuo, V.; Adogboba, D.; Ali, K.T.; Mashamba-Thompson, T.P. Geographic accessibility to public health facilities providing tuberculosis testing services at point-of-care in the upper east region, Ghana. BMC Public Health 2019, 19, 718. [Google Scholar] [CrossRef]
- Becher, H.; Müller, O.; Jahn, A.; Gbangou, A.; Kynast-Wolf, G.; Kouyaté, B. Risk factors of infant and child mortality in rural Burkina Faso. Bull. World Health Organ. 2004, 82, 265–273. [Google Scholar]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef]
- Anderle, A.; Bancone, G.; Domingo, G.J.; Gerth-Guyette, E.; Pal, S.; Satyagraha, A. Point-of-Care Testing for G6PD Deficiency: Opportunities for Screening. Int. J. Neonatal Screen. 2018, 4, 34. [Google Scholar] [CrossRef]
- Ley, B.; Luter, N.; Espino, F.E.; Devine, A.; Kalnoky, M.; Lubell, Y.; Thriemer, K.; Baird, J.K.; Poirot, E.; Conan, N.; et al. The challenges of introducing routine G6PD testing into radical cure: A workshop report. Malar. J. 2015, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Kuupiel, D.; Adu, K.; Bawontuo, V.; Adogboba, D.; Mashamba-Thompson, T.P. Estimating the Spatial Accessibility to Blood Group and Rhesus Type Point-of-Care Testing for Maternal Healthcare in Ghana. Diagnostics 2019, 9, 175. [Google Scholar] [CrossRef]
- Kuliszkiewicz-Janus, M.; Zimny, A. Glucose-6-phosphate dehydrogenase (G6PD) deficiency—A cause of anaemia in pregnant women. Pol. Arch. Intern. Med. 2003, 110, 1327–1333. [Google Scholar]
- WHO. Coronavirus Disease (COVID-19) Pandemic Geneva: World Health Organization. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 5 April 2020).
- Patel, A.; Waters, N. Using geographic information systems for health research. In Application of Geographic Information Systems; IntechOpen: London, UK, 2012. [Google Scholar]
- Dangisso, M.H.; Datiko, D.G.; Lindtjørn, B. Accessibility to tuberculosis control services and tuberculosis programme performance in southern Ethiopia. Glob. Health Action 2015, 8, 29443. [Google Scholar] [CrossRef] [PubMed]
- Kost, G.J. Theory, Principles, and Practice of Optimizing Point-of-Care Small-World Networks. Point Care J. Near Patient Test. Technol. 2012, 11, 96–101. [Google Scholar] [CrossRef]
- Ferguson, W.J.; Kemp, K.; Kost, G.J. Using a geographic information system to enhance patient access to point-of-care diagnostics in a limited-resource setting. Int. J. Health Geogr. 2016, 15, 10. [Google Scholar] [CrossRef]
- Kost, G.J. Geospatial Science and Point-of-Care Testing: Creating Solutions for Population Access, Emergencies, Outbreaks, and Disasters. Front. Public Health 2019, 7, 329. [Google Scholar] [CrossRef]
- Arthur, E. Wealth and antenatal care use: Implications for maternal health care utilisation in Ghana. Health Econ. Rev. 2012, 2, 14. [Google Scholar] [CrossRef]
- Gething, P.W.; Johnson, F.A.; Frempong-Ainguah, F.; Nyarko, P.; Baschieri, A.; Aboagye, P.; Falkingham, J.; Matthews, Z.; Atkinson, P.M. Geographical access to care at birth in Ghana: A barrier to safe motherhood. BMC Public Health 2012, 12, 991. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuupiel, D.; Adu, K.M.; Bawontuo, V.; Adogboba, D.A.; Drain, P.K.; Moshabela, M.; Mashamba-Thompson, T.P. Geographical Accessibility to Glucose-6-Phosphate Dioxygenase Deficiency Point-of-Care Testing for Antenatal Care in Ghana. Diagnostics 2020, 10, 229. https://doi.org/10.3390/diagnostics10040229
Kuupiel D, Adu KM, Bawontuo V, Adogboba DA, Drain PK, Moshabela M, Mashamba-Thompson TP. Geographical Accessibility to Glucose-6-Phosphate Dioxygenase Deficiency Point-of-Care Testing for Antenatal Care in Ghana. Diagnostics. 2020; 10(4):229. https://doi.org/10.3390/diagnostics10040229
Chicago/Turabian StyleKuupiel, Desmond, Kwame M. Adu, Vitalis Bawontuo, Duncan A. Adogboba, Paul K. Drain, Mosa Moshabela, and Tivani P. Mashamba-Thompson. 2020. "Geographical Accessibility to Glucose-6-Phosphate Dioxygenase Deficiency Point-of-Care Testing for Antenatal Care in Ghana" Diagnostics 10, no. 4: 229. https://doi.org/10.3390/diagnostics10040229
APA StyleKuupiel, D., Adu, K. M., Bawontuo, V., Adogboba, D. A., Drain, P. K., Moshabela, M., & Mashamba-Thompson, T. P. (2020). Geographical Accessibility to Glucose-6-Phosphate Dioxygenase Deficiency Point-of-Care Testing for Antenatal Care in Ghana. Diagnostics, 10(4), 229. https://doi.org/10.3390/diagnostics10040229






