Kynurenine/Tryptophan Ratio Predicts Angiotensin Receptor Blocker Responsiveness in Patients with Diabetic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Metabolomic Approach
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, C.E.; Christensen, C.K.; Vittinghus, E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983, 32, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, P.; Remuzzi, G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br. J. Clin. Pharmacol. 2013, 76, 516–523. [Google Scholar] [PubMed]
- Remuzzi, G.; Benigni, A.; Remuzzi, A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J. Clin. Investig. 2006, 116, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Donadelli, R.; Zanchi, C.; Morigi, M.; Buelli, S.; Batani, C.; Tomasoni, S.; Corna, D.; Rottoli, D.; Benigni, A.; Abbate, M.; et al. Protein overload induces fractalkine upregulation in proximal tubular cells through nuclear factor kappaB- and p38 mitogen-activated protein kinase-dependent pathways. J. Am. Soc. Nephrol. 2003, 14, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Gorriz, J.L.; Martinez-Castelao, A. Proteinuria: Detection and role in native renal disease progression. Transplant. Rev. 2012, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- de Zeeuw, D.; Remuzzi, G.; Parving, H.H.; Keane, W.F.; Zhang, Z.; Shahinfar, S.; Snapinn, S.; Cooper, M.E.; Mitch, W.E.; Brenner, B.M.; et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int. 2004, 65, 2309–2320. [Google Scholar] [CrossRef]
- Bakris, G.L. Slowing nephropathy progression: Focus on proteinuria reduction. Clin. J. Am. Soc. Nephrol. 2008, 3, S3–S10. [Google Scholar] [CrossRef]
- Kobori, H.; Mori, H.; Masaki, T.; Nishiyama, A. Angiotensin II blockade and renal protection. Curr. Pharm. Des. 2013, 19, 3033–3042. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Wang, G. Metabolomic biomarkers in diabetic kidney diseases–A systematic review. J. Diabetes Complicat. 2015, 29, 1345–1351. [Google Scholar] [CrossRef]
- Chou, C.A.; Lin, C.N.; Chiu, D.T.Y.; Chen, I.W.; Chen, S.T. Tryptophan as a surrogate prognostic marker for diabetic nephropathy. J. Diabetes Investig. 2018, 9, 366–374. [Google Scholar] [CrossRef]
- Molitch, M.E.; DeFronzo, R.A.; Franz, M.J.; Keane, W.F.; Mogensen, C.E.; Parving, H.H.; Steffes, M.W.; American Diabetes Association. Nephropathy in diabetes. Diabetes Care 2004, 27, S79–S83. [Google Scholar] [PubMed]
- Keane, W.F.; Eknoyan, G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the national kidney foundation. Am. J. Kidney Dis. 1999, 33, 1004–1010. [Google Scholar] [CrossRef]
- Jun, M.; Ohkuma, T.; Zoungas, S.; Colagiuri, S.; Mancia, G.; Marre, M.; Matthews, D.; Poulter, N.; Williams, B.; Rodgers, A.; et al. Changes in albuminuria and the risk of major clinical outcomes in diabetes: Results from ADVANCE-ON. Diabetes Care 2018, 41, 163–170. [Google Scholar] [CrossRef]
- Chamberlain, J.J.; Doyle-Delgado, K.; Peterson, L.; Skolnik, N. Diabetes technology: Review of the 2019 American diabetes association standards of medical care in diabetes. Ann. Intern. Med. 2019. [Google Scholar] [CrossRef]
- Iseki, K.; Iseki, C.; Ikemiya, Y.; Fukiyama, K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996, 49, 800–805. [Google Scholar] [CrossRef]
- Ruggenenti, P.P.A.; Mosconi, L.; Matalone, M.; Pisoni, R.; Gaspari, F.; Remuzzi, G. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int. Suppl. 1997, 63, S54–S57. [Google Scholar]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 2012, 23, 1917–1928. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef]
- Chuang, W.-H.; Arundhathi, A.; Lu, C.; Chen, C.-C.; Wu, W.-C.; Susanto, H.; Purnomo, J.D.T.; Wang, C.-H. Altered plasma acylcarnitine and amino acid profiles in type 2 diabetic kidney disease. Metabolomics 2016, 12. [Google Scholar] [CrossRef]
- Thomson, S.C.; Deng, A.; Bao, D.; Satriano, J.; Blantz, R.C.; Vallon, V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J. Clin. Investig. 2001, 107, 217–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the diabetic kidney. Diabetes Care 2016, 39, S165–S171. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.E.; Huber, R.G.; von Grafenstein, S.; Wallnoefer, H.G.; Spitzer, G.M.; Fuchs, D.; Liedl, K.R. Dynamic regulation of phenylalanine hydroxylase by simulated redox manipulation. PLoS ONE 2012, 7, e53005. [Google Scholar]
- Morgan, M.Y.; Marshall, A.W.; Milsom, J.P.; Sherlock, S. Plasma amino-acid patterns in liver disease. Gut 1982, 23, 362–370. [Google Scholar] [CrossRef]
- Saleem, T.; Dahpy, M.; Ezzat, G.; Abdelrahman, G.; Abdel-Aziz, E.; Farghaly, R. The profile of plasma free amino acids in type 2 diabetes mellitus with insulin resistance: Association with microalbuminuria and macroalbuminuria. Appl. Biochem. Biotechnol. 2019, 188, 854–867. [Google Scholar] [CrossRef]
- Debnath, S.; Velagapudi, C.; Redus, L.; Thameem, F.; Kasinath, B.; Hura, C.E.; Lorenzo, C.; Abboud, H.E.; O’Connor, J.C. Tryptophan metabolism in patients with chronic kidney disease secondary to type 2 diabetes: Relationship to inflammatory markers. Int. J. Tryptophan Res. 2017, 10. [Google Scholar] [CrossRef]
- Stone, T.W. Tryptophan and kynurenines: Continuing to court controversy. Clin. Sci. 2016, 130, 1335–1337. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef]
- Schrocksnadel, K.; Wirleitner, B.; Winkler, C.; Fuchs, D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta 2006, 364, 82–90. [Google Scholar] [CrossRef]
- Badawy, A.A. Tryptophan metabolism: A versatile area providing multiple targets for pharmacological intervention. Egypt. J. Basic Clin. Pharmacol. 2019, 9. [Google Scholar] [CrossRef]
- Badawy, A.A.; Guillemin, G. The plasma [Kynurenine]/[Tryptophan] ratio and indoleamine 2,3-dioxygenase: Time for appraisal. Int. J. Tryptophan Res. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, E.R.; Rebhun, S.; Eckel, M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J. Interferon Res. 1986, 6, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Tryptophan: The key to boosting brain serotonin synthesis in depressive illness. J. Psychopharmacol. 2013, 27, 878–893. [Google Scholar] [CrossRef] [PubMed]
- Imani, F.; Horii, Y.; Suthanthiran, M.; Skolnik, E.Y.; Makita, Z.; Sharma, V.; Sehajpal, P.; Vlassara, H. Advanced glycosylation endproduct-specific receptors on human and rat T-lymphocytes mediate synthesis of interferon gamma: Role in tissue remodeling. J. Exp. Med. 1993, 178, 2165–2172. [Google Scholar] [CrossRef]
- Zhao, J. Plasma kynurenic acid/tryptophan ratio: A sensitive and reliable biomarker for the assessment of renal function. Ren. Fail. 2013, 35, 648–653. [Google Scholar] [CrossRef]
- Izuhara, Y.; Nangaku, M.; Inagi, R.; Tominaga, N.; Aizawa, T.; Kurokawa, K.; van Ypersele de Strihou, C.; Miyata, T. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J. Am. Soc. Nephrol. 2005, 16, 3631–3641. [Google Scholar] [CrossRef]
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann. Intern. Med. 2001, 134, 370–379. [Google Scholar] [CrossRef]
- Strippoli, G.F.; Bonifati, C.; Craig, M.; Navaneethan, S.D.; Craig, J.C. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Kshirsagar, A.V.; Joy, M.S.; Hogan, S.L.; Falk, R.J.; Colindres, R.E. Effect of ACE inhibitors in diabetic and nondiabetic chronic renal disease: A systematic overview of randomized placebo-controlled trials. Am. J. Kidney Dis. 2000, 35, 695–707. [Google Scholar] [CrossRef]
- Hovind, P.; Rossing, P.; Tarnow, L.; Toft, H.; Parving, J.; Parving, H.H. Remission of nephrotic-range albuminuria in type 1 diabetic patients. Diabetes Care 2001, 24, 1972–1977. [Google Scholar] [CrossRef]
- Rossing, K.; Christensen, P.K.; Hovind, P.; Tarnow, L.; Rossing, P.; Parving, H.H. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004, 66, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Araki, S.; Honjo, J.; Okizaki, S.; Yamada, D.; Shudo, R.; Shimizu, H.; Sone, H.; Moriya, T.; Haneda, M.; et al. Association between remission of macroalbuminuria and preservation of renal function in patients with type 2 diabetes with overt proteinuria. Diabetes Care 2013, 36, 3227–3233. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.X.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I.; et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef]
- Zakrocka, I.; Targowska-Duda, K.M.; Wnorowski, A.; Kocki, T.; Jozwiak, K.; Turski, W.A. Angiotensin II type 1 receptor blockers inhibit KAT II activity in the brain-Its possible clinical applications. Neurotox. Res. 2017, 32, 639–648. [Google Scholar] [CrossRef]
- de Jong, P.E.; Gansevoort, R.T.; Bakker, S.J. Macroalbuminuria and microalbuminuria: Do both predict renal and cardiovascular events with similar strength? J. Nephrol. 2007, 20, 375–380. [Google Scholar]
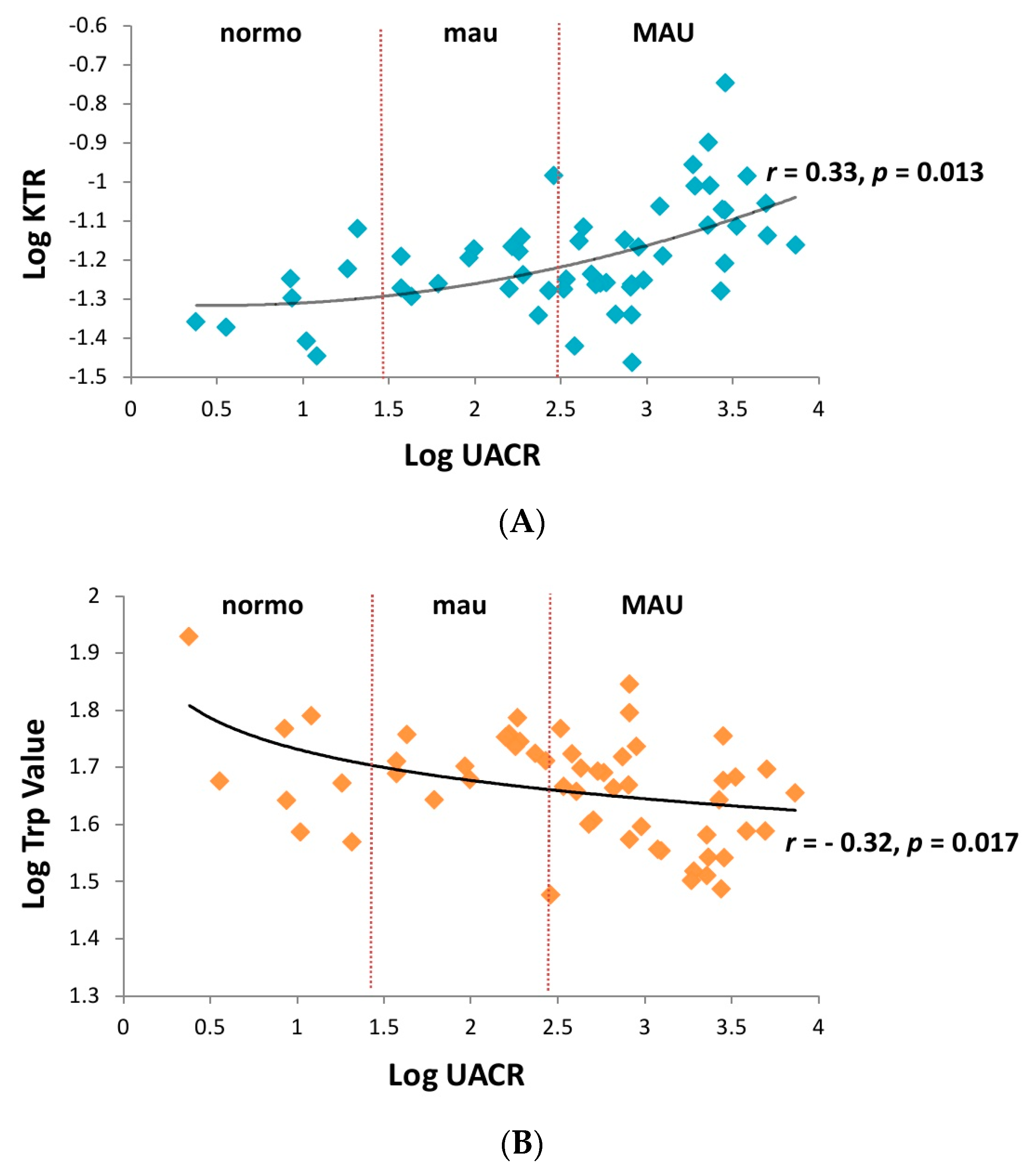

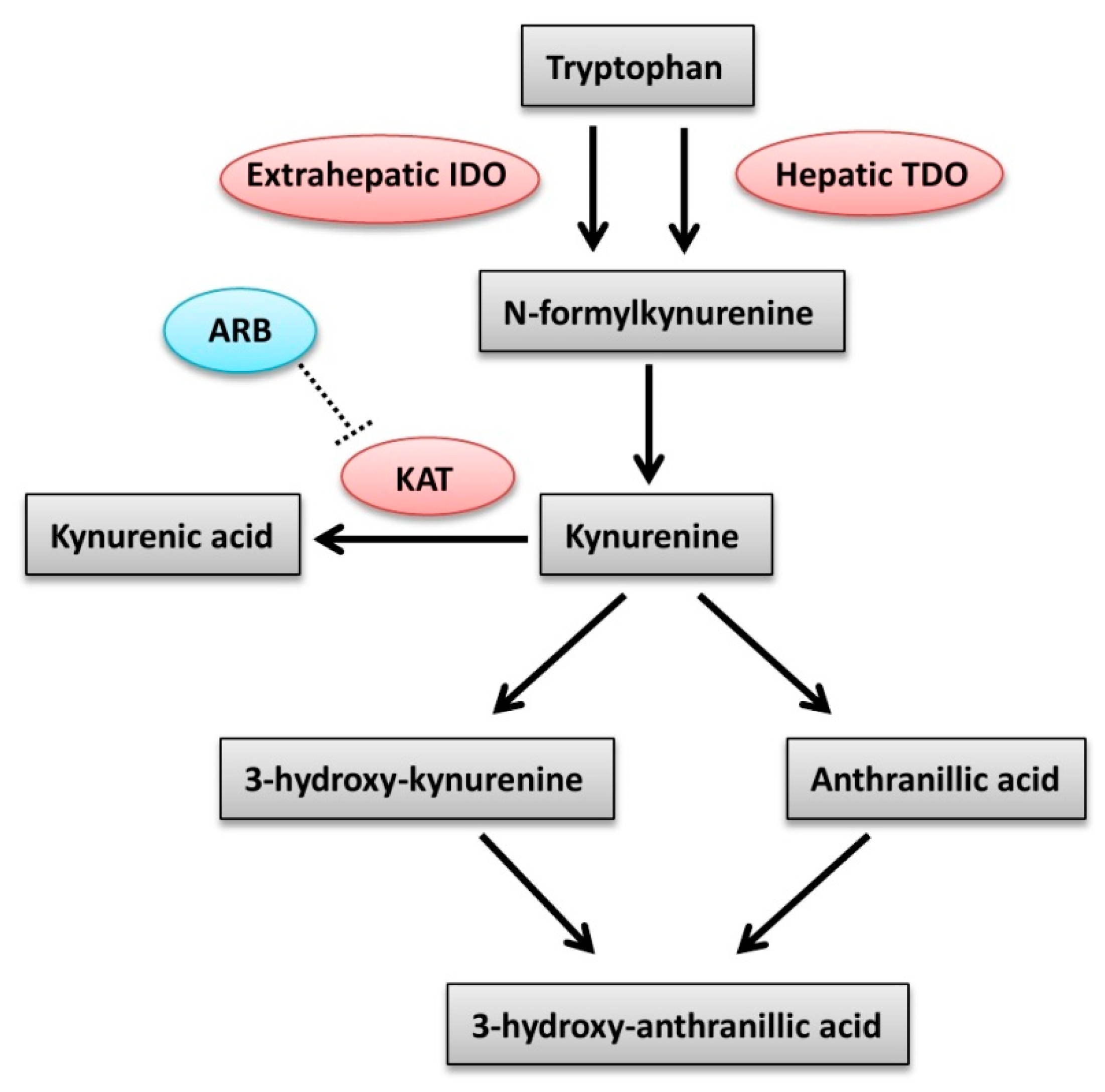
| Variable | MAU (n = 34) | Mau (n = 14) | Control (n = 8) | p Value |
|---|---|---|---|---|
| Gender | ||||
| Male | 20 (58.8) | 6 (42.9) | 4 (50.0) | 0.597 |
| Female | 14 (41.2) | 8 (57.1) | 4 (50.0) | |
| Age (years) | 61.5 ± 8.5 | 68.1 ± 5.6 | 66.9 ± 4.6 | 0.013 * |
| BMI (kg/m2) | 27.9 ± 4.8 | 26.4 ± 5.2 | 25.4 ± 3.4 | 0.341 |
| HbA1c (%) | 7.73 ± 1.31 | 7.29 ± 0.74 | 7.34 ± 0.44 | 0.393 |
| Duration of DM (years) | 13.9 ± 7.3 | 13.3 ± 5.7 | 14.8 ± 6.8 | 0.890 |
| SBP (mmHg) | 134.7 ± 10.9 | 134.9 ± 8.35 | 137.1 ± 11.0 | 0.833 |
| DBP (mmHg) | 78.0 ± 11.5 | 76.3 ± 6.12 | 77.8 ± 11.6 | 0.873 |
| UACR (mg/g) ‡ | 1831.0 ± 1640.5 | 146.5 ± 85.9 | 10.6 ± 6.4 | <0.001 * |
| Cr (mg/dL) ‡ | 1.92 ± 1.32 | 1.27 ± 0.26 | 1.33 ± 0.30 | 0.103 |
| eGFR (mL/min/1.73 m2) ‡ | 42.9 ± 18.6 | 50.1 ± 12.8 | 49.2 ± 12.8 | 0.325 |
| CKD stage II | 4 (11.8) | 4 (28.6) | 1 (12.5) | 0.314 |
| CKD stage III | 22 (64.7) | 9 (64.3) | 7 (87.5) | |
| CKD stage IV, V | 8 (23.5) | 1 (7.1) | 0 | |
| OAD with | ||||
| Metformin | 14 (41.2) | 7 (50.0) | 6 (75.0) | 0.260 |
| Sulfonylurea | 24 (70.6) | 10 (71.4) | 6 (75.0) | 1.000 |
| DPP4 inhibitor | 15 (44.1) | 9 (64.3) | 5 (62.5) | 0.412 |
| GLP-1R agonist SGLT2 inhibitor | 4 (11.2) 5 (14.7) | 0 0 | 0 0 | 0.332 0.308 |
| Insulin injection | 13 (38.2) | 3 (21.4) | 2 (25.0) | 0.603 |
| Anti-hypertensive drugs | ||||
| Beta-blacker | 8 (23.5) | 7 (50.0) | 2 (25.0) | 0.195 |
| CCB | 22 (64.7) | 10 (71.4) | 4 (50.0) | 0.569 |
| Diuretics | 4 (11.8) | 3 (21.4) | 0 | 0.376 |
| Metabolites | MAU (n = 34) | Mau (n = 14) | Control (n = 8) | p Value |
|---|---|---|---|---|
| Amino acids | ||||
| Ser | 99.9 ± 25.0 | 119.9 ± 31.8 | 126.0 ± 24.8 | 0.016 * |
| Trp | 44.5 ± 9.32 | 51.3 ± 7.62 | 52.3 ± 15.7 | 0.042 * |
| Tyr | 53.3 ± 10.8 | 66.1 ± 7.57 | 64.6 ± 16.7 | 0.001 * |
| Orn ‡ | 122.7 ± 35.3 | 158.1 ± 68.2 | 95.9 ± 31.8 | 0.020 * |
| Phe ‡ | 65.7 ± 13.6 | 75.1 ± 10.3 | 66.9 ± 13.2 | 0.007 * |
| Biogenic amines | ||||
| Kyn | 3.10 ± 0.89 | 3.14 ± 0.56 | 2.56 ± 0.72 | 0.207 |
| Kyn/Trp | 0.073 ± 0.028 | 0.062 ± 0.014 | 0.050 ± 0.013 | 0.046 * |
| Glycerophospholipids | ||||
| PC ae C44:3 ‡ | 0.102 ± 0.022 | 0.118 ± 0.028 | 0.126 ± 0.025 | 0.025 * |
| lysoPC a C24:0 | 0.138 ± 0.032 | 0.164 ± 0.031 | 0.166 ± 0.034 | 0.013 * |
| lysoPC a C26:1 ‡ | 0.027 ± 0.009 | 0.034 ± 0.008 | 0.034 ± 0.008 | 0.006 * |
| Sphingolipids | ||||
| SM C26:0 | 0.208 ± 0.047 | 0.242 ± 0.062 | 0.249 ± 0.041 | 0.036 * |
| Metabolites | MAU Group | Metabolites | Mau Group | ||||
|---|---|---|---|---|---|---|---|
| Responder (n = 20) | Non-Responder (n = 14) | p Value | Responder (n = 7) | Non-Responder (n = 7) | p Value | ||
| Amino acids | Amino acids | ||||||
| Ser | 95.7 ± 25.7 | 105.1 ± 24.1 | 0.306 | Ser | 129.3 ± 41.3 | 110.4 ± 16.5 | 0.296 |
| Trp | 42.4 ± 6.84 | 47.6 ± 11.6 | 0.108 | Trp ‡ | 52.2 ± 4.75 | 50.3 ± 10.1 | 0.749 |
| Tyr | 53.7 ± 10.3 | 52.8 ± 11.9 | 0.805 | Tyr | 66.1 ± 5.10 | 66.1 ± 9.91 | 1.000 |
| Orn ‡ | 126.3 ± 29.7 | 118.3 ± 41.9 | 0.351 | Orn | 165.6 ± 57.2 | 150.6 ± 81.6 | 0.698 |
| Phe ‡ | 68.4 ± 15.6 | 61.8 ± 9.10 | 0.178 | Phe ‡ | 78.3 ± 11.6 | 71.9 ± 8.51 | 0.142 |
| Biogenic amines | Biogenic amines | ||||||
| Kyn ‡ | 3.32 ± 0.97 | 2.79 ± 0.67 | 0.112 | Kyn | 2.93 ± 0.59 | 3.34 ± 0.48 | 0.175 |
| Kyn/Trp ‡ | 0.081 ± 0.031 | 0.060 ± 0.015 | 0.025 * | Kyn/Trp ‡ | 0.056 ± 0.008 | 0.069 ± 0.017 | 0.085 |
| Glycerophospholipids | Glycerophospholipids | ||||||
| PC ae C44:3 ‡ | 0.103 ± 0.025 | 0.100 ± 0.018 | 0.834 | PC ae C44:3 | 0.116 ± 0.036 | 0.121 ± 0.020 | 0.746 |
| lysoPC a C24:0 | 0.135 ± 0.028 | 0.141 ± 0.037 | 0.608 | lysoPC a C24:0 | 0.153 ± 0.029 | 0.175 ± 0.030 | 0.187 |
| lysoPC a C26:1 ‡ | 0.027 ± 0.010 | 0.026 ± 0.006 | 0.888 | lysoPC a C26:1 | 0.032 ± 0.009 | 0.036 ± 0.007 | 0.297 |
| Sphingolipids | Sphingolipids | ||||||
| SM C26:0 | 0.209 ± 0.054 | 0.208 ± 0.038 | 0.969 | SM C26:0 | 0.254 ± 0.079 | 0.231 ± 0.040 | 0.494 |
| Models | Multivariate Odds Ratio (95% Confidence Interval) | p Value |
|---|---|---|
| Unadjusted model | 0.639 (0.415-0.983) | 0.041 * |
| Model 1 (age) | 0.644 (0.417-0.994) | 0.047 * |
| Model 2 (SBP) | 0.619 (0.386-0.991) | 0.046 * |
| Model 3 (eGFR) | 0.377 (0.148-0.964) | 0.042 * |
| Model 4 (gender) | 0.319 (0.112-0.907) | 0.032 * |
| Model 5 (HbA1c) | 0.326 (0.112-0.951) | 0.040 * |
| Model 6 (duration of diabetes) | 0.218 (0.057-0.834) | 0.026 * |
| Model 7 (use of DPP4 inhibitor or GLP-1R agonist or SGLT-2 inhibitor) | 0.098 (0.012-0.814) | 0.032 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-H.; Lin, C.-N.; Chiu, D.T.-Y.; Chen, S.-T. Kynurenine/Tryptophan Ratio Predicts Angiotensin Receptor Blocker Responsiveness in Patients with Diabetic Kidney Disease. Diagnostics 2020, 10, 207. https://doi.org/10.3390/diagnostics10040207
Wu M-H, Lin C-N, Chiu DT-Y, Chen S-T. Kynurenine/Tryptophan Ratio Predicts Angiotensin Receptor Blocker Responsiveness in Patients with Diabetic Kidney Disease. Diagnostics. 2020; 10(4):207. https://doi.org/10.3390/diagnostics10040207
Chicago/Turabian StyleWu, Ming-Hsien, Chia-Ni Lin, Daniel Tsun-Yee Chiu, and Szu-Tah Chen. 2020. "Kynurenine/Tryptophan Ratio Predicts Angiotensin Receptor Blocker Responsiveness in Patients with Diabetic Kidney Disease" Diagnostics 10, no. 4: 207. https://doi.org/10.3390/diagnostics10040207
APA StyleWu, M.-H., Lin, C.-N., Chiu, D. T.-Y., & Chen, S.-T. (2020). Kynurenine/Tryptophan Ratio Predicts Angiotensin Receptor Blocker Responsiveness in Patients with Diabetic Kidney Disease. Diagnostics, 10(4), 207. https://doi.org/10.3390/diagnostics10040207





